Chromatogram profile of andrographolide-glucuronate and andrographolide glycine in rabbit feces extract
Abstract
Pharmacokinetic studies of plant extract have been carried out worldwide. Our previous pharmacokinetic study of andrographolide, a major diterpenoid of Andrographis paniculata leaves given orally in inflammation-induced New Zealand rabbits, demonstrated that this compound was excreted in the form of free andrographolide and an unknown metabolite. This work aimed to study the RP-HPLC profile of andrographolide-glucuronic acid and andrographolide-glycine in rabbit feces by reacting equimols of the reactants (5 mg of andrographolide with 2.77 mg of glucuronic acid and 1.07 mg of glycine, in separate reaction, spiked into 10 g of rabbit feces), liquid-liquid extracted using water-ethylacetate (1:1) at 45-50 oC for 2 h, and subsequently, the extract was injected in a C18 column (particle size 5 µm, pore size 120 Å) with methanol-water (55:45) as the mobile phase. Moreover, ligand-ligand docking simulation also studied the binding mode of andrographolide with glucuronic acid and andrographolide with glycine. Results revealed that the maximum absorbance of standard andrographolide was detected at 227 nm and this compound was eluted at 6.120 min, glucuronic acid was eluted at 1.840 min, and glycine was at 2.207 min. The HPLC chromatogram of andrographolide-glucuronic acid extract demonstrated almost negligible peaks at 6.113 min and 6.107 min, which probably belong to each of the complexes, respectively. Moreover, andrographolide interacts with glucuronic acid via 1 hydrogen bond, although not favorable, and interacts with glycine via 2 hydrogen bonds. Taken together, andrographolide might undergo phase 2 biotransformation by glucuronidation and/or glycine conjugation in rabbits.
Keywords: Andrographolide, Anti-inflammatory, Biotransformation, In vitro study, Molecular docking simulation
Introduction
Pharmacokinetic studies of plant extract have been carried out worldwide [1-3]. In fact, carrying out a pharmacokinetic study is not as easy as expected, due to various metabolic processes and enzymatic hydrolysis that are encountered by the bioactive compound of the extracts in the body.
Andrographolide, a major diterpenoid compound of Andrographis paniculata, has been extensively explored for its pharmacology activities and pharmacokinetics [4-10]. Oral administration of a non-steroid anti-inflammatory drug together with andrographolide in rabbits has improved the ADME of this diterpenoid compound [4]. An earlier biodistribution study revealed that the iodo-andrographolide radiopharmaceutics (131I-andrographolide) was distributed in all organs with the highest accumulation occurring in the stomach and the thyroid of lipopolysaccharide-induced mice [6]. Moreover, a recent study reported that [11]. F‐andrographolide was also distributed in all organs with a majority accumulation in the liver of acute pneumonia Kunming mice [7].
Andrographolide could alter and/or enhance the bioavailability of other drugs when given simultaneously. A study in Weifang, China, reported that co-administration of andrographolide significantly affected the pharmacokinetics of warfarin in male Sprague-Dawley rats [12]. Another study in Taiwan described that andrographolide could hasten the metabolism rate of tolbutamide in male Sprague-Dawley rats. In this study, andrographolide underwent phase II biotransformation by reacting with UDP-glucuronosyltransferase and glutathione S-transferase [13]. Seven metabolites of andrographolide in human urine have been characterized as glucuronide conjugates [14]. The biotransformation reaction of andrographolide and UDP-glucuronosyltransferase was also described previously (Figure 1) [15].
|
|
|
Figure 1. The glucuronidation reaction of andrographolide [15]. |
Therefore, it is interesting to study the RP-HPLC profile of andrographolide-glucuronic acid and andrographolide-glycine in rabbit feces by employing methanol-water as the mobile phase. Moreover, molecular docking simulation also studied the binding mode of andrographolide with glucuronic acid and andrographolide with glycine.
Experimental
Chemicals
Andrographolide 98% 500 mg CAS 5508-58-7 for R & D use (Sigma Aldrich®), glucuronic acid (Cat. No. G5269, Sigma Aldrich®), glycine for analysis (Cat. No. 1042010100, Merck), double-distilled water for HPLC analysis (Ikapharmindo Putramas, Indonesia), ethanol 96% technical grade (BrataChem, Indonesia), ethyl acetate technical grade (BrataChem, Indonesia), chloroform technical grade (BrataChem, Indonesia), methanol for HPLC analysis (JT Baker®).
Animals
The animal study protocol was approved by the Research Ethics Committee of Universitas Padjadjaran (letter no. 047/UN6.C1.3.2/KEPK/PN/2016). The animals used were six (3 males and 3 females) healthy New Zealand rabbits (Figure 2) (bred at the Rajawali Rabbit Farm), aged 4-6 mo, with a weight of 1.5-2.5 kg. Each rabbit was collected for its feces in 2x24 h. The rabbits were acclimatized for 7 d before treatment and were given standard feed and sufficient water. The rabbits were confirmed healthy by the measurement of their body temperature (anal), respiration rate, body weight, and heart rate. Their feed consumption was also observed.
Ultraviolet spectrophotometry to determine the maximum wavelength of andrographolide
5 mg of andrographolide (Cat. No. 365645, Sigma Aldrich®) was dissolved in 100 mL of methanol (50 μg/mL). The solution was diluted using the same solvent to obtain a 12 μg/mL concentration. The maximum wavelength of 12 μg/mL andrographolide in methanol was determined using an ultraviolet spectrophotometer and resulted in λmax of 227 nm with an absorbance of 0.5407.
Determination of the retention time of standard andrographolide, glucuronic acid, and glycine in methanol using reversed-phase high-performance liquid chromatography
20 μL of each standard andrographolide (12 μg/mL), glucuronic acid (100 μg/mL), and glycine (100 μg/mL) in methanol, was injected in a C18 column (particle size 5 µm, pore size 120 Å) with methanol-water (55:45) as the mobile phase. The flow rate was 0.5 mL/min and detection set at 227 nm.
|
|
|
(a) |
|
|
|
(b) |
|
Figure 2. The measurement of body temperature (a) and heart rate (b) of the New Zealand rabbits |
Feces collection and preparation of the sample
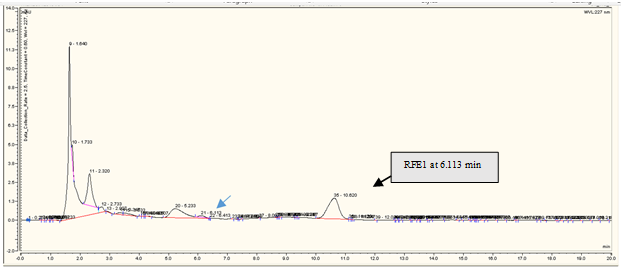
The feces were collected and weighed @10 g to be mixed with each reaction: reaction 1 (5 mg of andrographolide with 2.77 mg of glucuronic acid) and reaction 2 (5 mg of andrographolide with 1.07 mg of glycine). The mixtures were homogenized and extracted using methanol at 45-50 oC. The solvents were evaporated in a rotavapor at 45 oC, 75 rpm. The viscous extract was concentrated in a water bath at 50 oC. The residue was subjected to liquid-liquid extraction using a mixture of water and ethyl acetate (1:1). The water fraction was separated and centrifugated at 2,000 rpm for 30 min. The supernatant was further processed in a freeze-dryer. The solid residue was dissolved in methanol for HPLC analysis and labeled as the RFE1 and RFE2.
Reversed-Phase high-performance liquid chromatography to determine the chromatogram profile of andrographolide-glucuronic acid and andrographolide-glycine in rabbit feces
20 µL of each RFE1 and RFE2 extract were injected in a C18 column (particle size 5 µm, pore size 120 Å) with methanol-water (55:45) as the mobile phase. The flow rate was 0.5 mL/min and detection set at 227 nm.
Preparation and energy optimization of andrographolide, glucuronic acid, and glycine using chem
2D structures of andrographolide, glucuronic acid, and glycine were generated using ChemBioDraw Ultra14.0 free trial (downloaded from www.cambridgesoft.com) and were geometry optimized by employing AM1 (Austin Model 1) to produce accurate geometric structures.
Ligand-ligand docking simulation
Ligand-ligand docking simulation was carried out for andrographolide and glucuronic acid using AutoDock 4.2.6. The same procedure was done for andrographolide and glycine. The procedure was the adoption of previous techniques described elsewhere by a few modifications [5, 16-18].
Results and Discussion
During 7 days of acclimatization, the rabbits were confirmed as healthy, and their condition is presented in Table 1.
|
Table 1. Preliminary Health Examination of the Rabbits |
|||||
|
Rabbit no. |
BW (kg) |
Body Temperature (oC) |
Respiration Rate (per minute) |
Heart Rate (per minute) |
Condition |
|
1 |
1.74 |
37.33 |
60 |
131 |
Healthy |
|
2 |
1.71 |
38.01 |
68 |
147 |
Healthy |
|
3 |
1.71 |
38.28 |
58 |
147 |
Healthy |
|
4 |
1.74 |
38.28 |
62 |
139 |
Healthy |
|
5 |
2.38 |
38.81 |
62 |
134 |
Healthy |
|
6 |
2.21 |
38.46 |
65 |
144 |
Healthy |
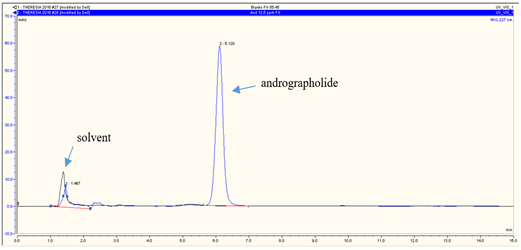
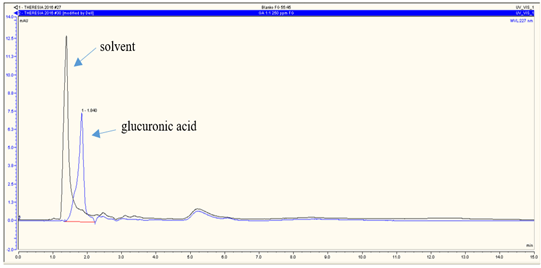
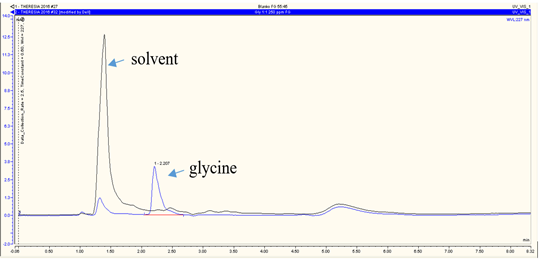
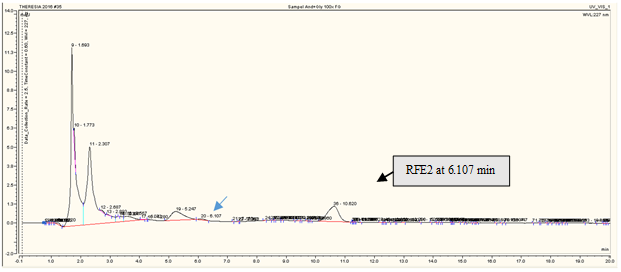
RP-HPLC analysis revealed that andrographolide was eluted at 6.120 min (Figure 3a), glucuronic acid was eluted at 1.840 min (Figure 3b), and glycine was at 2.207 min (Figure 3c). The overall chromatograms are presented in Figure 3. The RP-HPLC chromatogram of the RFE1 and RFE2 extracts demonstrated almost negligible peaks at 6.113 min (Figure 4a) and 6.107 min (Figure 4b), which probably belongs to RFE1 (andrographolide-glucuronic acid) extract and RFE2 (andrographolide-glycine) extract, respectively.
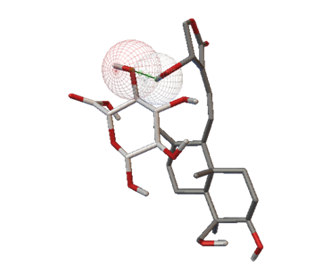
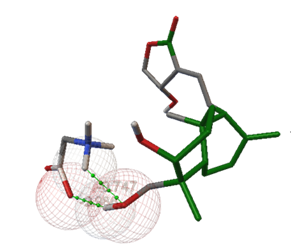
Moreover, ligand-ligand docking of andrographolide-glucuronic acid (Figures 5a and 5b) and andrographolide-glycine (Figures 5c and 5d) demonstrated that andrographolide more easily interacts with glycine than glucuronic acid. The hydroxyl (-OH) group at position C-14 of andrographolide interacts with the -OH of glucuronic acid via the formation of one hydrogen bond (bond distance 1.319 Å). Interestingly, the -OH group at position C-19 of andrographolide interacts with the -NH2 and the carbonyl (C=O) of glycine via the formation of two hydrogen bonds (bond distance 2.082 Å and 1.839 Å, respectively).
Glucuronidation of andrographolide using liver microsomes and recombinant UGT enzymes was previously reported by Tian and co-workers. Not similar to our in silico study, their in vitro study revealed that glucuronidation of andrographolide occurred at C-3 and C-19 [15]. Andrographolide stimulated the cytochrome enzyme by activating the binding of the Aryl hydrocarbon Receptor (AhR) and Pregnane X receptor (PXR) to DNA [8]. The expression of the CYP1A1 gene is managed by the activation of AhR upon the binding of a ligand. Interestingly, andrographolide is a potent modulator of the expression and activity of CYP enzymes [11].
Taken together, the pharmacokinetic study of various phytoconstituents, especially when combined with drugs, has been proven to be the utmost challenge. Herbal medicines containing A. paniculata extract were reported could alter the elimination of theophylline, thus asthma patients treated with CYP1A2-metabolized drugs, e.g. theophylline, should be advised of the tendency of herb-drug interaction [19]. Andrographolide is excreted either as unchanged andrographolide or as its conjugated forms [20]. However, conjugation with sulfate was reported to occur at C-3 of andrographolide [21]. A pharmacokinetic study of 14-deoxy-12-hydroxy-andrographolide, a metabolite of andrographolide, after oral administration of andrographolide in rats revealed that the metabolite was absorbed faster than andrographolide, as proven by its early presence in the animal’s blood [22]. Moreover, a pharmacokinetic study of 14-deoxy-11, 12-didehydroandrographolide, neoandrographolide, and 14-deoxyandrographolide in Thai healthy humans reported that the maximum plasma concentration (Cmax) and the area under curve (AUC) of the four diterpenoids are significantly different between males and females participants [23]. However, one should be aware that the co-administration of medicinal herbs, in this case, A. paniculata, may alter a drug’s pharmacokinetics and even arise toxicity. As reported previously, the co-administration of A. paniculata extract diminished the pharmacokinetic parameters (T1/2) of glipizide [24].
|
|
|
(a) |
|
|
|
(b) |
|
|
|
(c) |
|
Figure 3. RP-HPLC chromatogram of standard (a) andrographolide; (b) glucuronic acid; and (c) glycine. Stationary phase: C18 column, particle size 5 µm, pore size 120 Å; mobile phase: CH3OH-H2O 55:45; flow rate of 0.5 mL/min |
|
|
|
(a) |
|
|
|
(b) |
|
Figure 4. RP-HPLC chromatogram of (a) andrographolide-glucuronic acid (RFE1) and (b) andrographolide-glycine (RFE2) in rabbit feces extract. Stationary phase: C18 column, particle size 5 µm, pore size 120 Å; mobile phase: CH3OH-H2O 55:45; flow rate of 0.5 mL/min. |
|
|
|
|
(a) |
(b) |
|
|
|
|
(c) |
(d) |
|
Figure 5. Ligand-ligand docking simulation of (a) andrographolide-glucuronic acid in 3D visualization; (b) andrographolide-glucuronic acid in 2D visualization; (c) andrographolide-glycine in 3D visualization; (d) andrographolide-glycine in 2D visualization. |
|
Conclusion
This study discloses that andrographolide analyzed by RP-HPLC was eluted at 6.120 min, glucuronic acid was eluted at 1.840 min, and glycine was at 2.207 min. The chromatogram of andrographolide-glucuronic acid extract and andrographolide-glycine extract of rabbit feces demonstrated almost negligible peaks at 6.113 min and 6.107 min, which probably belong to each of the complexes, respectively. Ligand-ligand docking simulation confirmed that andrographolide interacts with glucuronic acid via one hydrogen bond at 1.319 Å, although not favorably, and interacts with glycine via two hydrogen bonds at 2.082 Å and 1.389 Å. Taken together, andrographolide might undergo phase 2 biotransformation by glucuronidation and/or glycine conjugation in rabbits.
Acknowledgments: The authors thank the Rector of Universitas Padjadjaran via the Directorate of Research and Community Engagement for funding the publication fee of this article via the Internal Research Academic-Leadership Grant of Prof. Dr. Jutti Levita.
Conflict of interest: None
Financial support: The research and the publication fee are funded by Universitas Padjadjaran in the scheme of the Universitas Padjadjaran Internal Research Academic-Leadership Grant of Prof. Dr. Jutti Levita.
Ethics statement: The animal study protocol was approved by the Research Ethics Committee of Universitas Padjadjaran (letter no. 047/UN6.C1.3.2/KEPK/PN/2016).
References
- Panossian A, Hovhannisyan A, Mamikonyan G, Abrahamian H, Hambardzumyan E, Gabrielian E, et al. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine. 2000;7(5):351-4. doi:10.1016/S0944-7113(00)80054-9
- Sun S, Wang Y, Wu A, Ding Z, Liu X. Influence Factors of the Pharmacokinetics of Herbal Resourced Compounds in Clinical Practice. Evid Based Complement Alternat Med. 2019;2019:1983780. doi:10.1155/2019/1983780
- Levita J, Syafitri DM, Supu RD, Mutakin M, Megantara S, Febrianti M, et al. Pharmacokinetics of 10 gingerols and 6 shogaol in the plasma of healthy subjects treated with red ginger (Zingiber officinale var. Rubrum) suspension. Biomed Rep. 2018;9(6):474-82. doi:10.3892/br.2018.1163
- Mutakin M, Megantara S, Larasati BA, Yogiyanto Y, Levita J, Ibrahim S. The pharmacokinetic drug-drug interactions of Andrographis paniculata and ibuprofen in the plasma of healthy Oryctolagus cuniculus rabbits. PCPR. 2020;5(2):40. doi:10.15416/pcpr.v5i2.27508
- Megantara S, Levita J, Ibrahim S, Nguyen BP. Andrographolide, a diterpenoid lactone compound of Andrographis paniculata, binds to Lys353 and Asp38 in the peptidase domain of human angiotensin-converting enzyme 2. Rasayan J Chem. 2021;14(1):241. doi:10.31788/RJC.2021.1416070
- Levita J, Ardiyatno CN, Nawawi A, Mutalib A, Ibrahim S. Radioiodination of andrographolide and its biodistribution in mice for the inflammatory tracer. Indonesian J Pharm. 2010;21(4):258. doi:10.14499/indonesianjpharm0iss0pp258-265
- Zhang Q, Cui Q. Biodistribution of andrographolide to assess the interior-exterior relationship between the lung and intestine using micro PET. Thorac Cancer. 2020;11(11):3365-74. doi:10.1111/1759-7714.13682
- Chen HW, Huang CS, Liu PF, Li CC, Chen CT, Liu CT, et al. Andrographis paniculata extract and andrographolide modulate the hepatic drug metabolism system and plasma tolbutamide concentrations in rats. Evid Based Complement Alternat Med. 2013;2013:982689. doi:10.1155/2013/982689
- Levita J, Juwita T, Ramadhani S, Saptarini NM, Mutakin M. Chromatogram profiles of andrographolide in A23187-induced New Zealand rabbit’s urine and feces. J Appl Pharm Sci. 2017;7(01):156. doi:10.7324/JAPS.2017.70121
- Zhao HY, Hu H, Wang YT. Comparative metabolism and stability of andrographolide in liver microsomes from humans, dogs, and rats using ultra-performance liquid chromatography coupled with triple-quadrupole and Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom. 2013;27(12):1385. doi:10.1002/rcm.6585
- Kondo S, Chatuphonprasert W, Jaruchotikamol A, Sakuma T, Nemoto N. Cellular glutathione content modulates the effect of andrographolide on β-naphthoflavone-induced CYP1A1 mRNA expression in mouse hepatocytes. Toxicology. 2011;280(1-2):18-23. doi:10.1016/j.tox.2010.11.002
- Zhang X, Zhang X, Wang X, Zhao M. Influence of andrographolide on the pharmacokinetics of warfarin in rats. Pharm Biol. 2018;56(1):351. doi:10.1080/13880209.2018.1478431
- Chen HW, Huang CS, Liu PF, Li CC, Chen CT, Liu CT, et al. Andrographis paniculata extract and andrographolide modulate the hepatic drug metabolism system and plasma tolbutamide concentrations in rats. Evid Based Complement Alternat Med. 2013;2013:982689. doi:10.1155/2013/982689
- Cui L, Qiu F, Yao X. Isolation and identification of seven glucuronide conjugates of andrographolide in human urine. Drug Metabolism and Disposition. 2005;33:555. doi:10.1124/dmd.104.001958
- Tian X, Liang S, Wang C, Wu B, Ge G, Deng S, et al. Erratum to: regioselective glucuronidation of andrographolide and its major derivatives: metabolite identification, isozyme contribution, and species differences. AAPS J. 2015;17(2):479.
- Megantara S, Mutakin M, Halimah E, Febrina E, Levita J. Molecular interaction of the downstream executioner cysteine aspartyl proteases (caspase-3 and caspase-7) with corilagin, quercetin, rutin, kaempferol, gallic acid, and geraniin of Acalypha wilkesiana Müll. Arg. Rasayan J Chem. 2020;13(3):1321. doi:10.31788/%20RJC.2020.1335766
- Aulifa DL, Adnyana IK, Levita J, Sukrasno S. 4-Hydroxyderricin isolated from the sap of Angelica keiskei Koidzumi: Evaluation of its inhibitory activity towards dipeptidyl peptidase-IV. Sci Pharm. 2019;87(4):30. doi:10.3390/scipharm87040030
- Lolok N, Sumiwi SA, Muhtadi A, Susilawati Y, Hendriani R, Ramadhan DSF, et al. Molecular docking and molecular dynamics studies of bioactive compounds contained in noni fruit (Morinda citrifolia L.) against human pancreatic α-amylase. J Biomol Struct Dyn. 2021;40(15):7091. doi:10.1080/07391102.2021.1894981
- Chien CF, Wu YT, Lee WC, Lin LC, Tsai TH. Herb-drug interaction of Andrographis paniculata extract and andrographolide on the pharmacokinetics of theophylline in rats. Chem Biol Interact. 2010;184(3):458-65. doi:10.1016/j.cbi.2010.01.017
- Bera R, Ahmed SK, Sarkar L, Sen T, Karmakar S. Pharmacokinetic analysis and tissue distribution of andrographolide in the rat by a validated LC-MS/MS method. Pharm Biol. 2014;52(3):321. doi:10.3109/13880209.2013.836544
- He X, Li J, Gao H, Qiu F, Cui X, Yao X. Six new andrographolide metabolites in rats. Chem Pharm Bull (Tokyo). 2003;51(5):586. doi:10.1248/cpb.51.586
- Yang T, Xu C, Wang ZT, Wang CH. Comparative pharmacokinetic studies of andrographolide and its metabolite of 14-deoxy-12-hydroxy-andrographolide in rat by ultra-performance liquid chromatography-mass spectrometry. Biomed Chromatogr. 2013;27(7):931. doi:10.1002/bmc.2884
- Pholphana N, Panomvana D, Rangkadilok N, Suriyo T, Puranajoti P, Ungtrakul T, et al. Andrographis paniculata: Dissolution investigation and pharmacokinetic studies of four major active diterpenoids after multiple oral dose administration in healthy Thai volunteers. J Ethnopharmacol. 2016;194:513. doi:10.1016/j.jep.2016.09.058
- Sundhani E, Nugroho AE, Nurrochmad A, Puspitasari I, Amalia Prihati D, Lukitaningsih E. Pharmacokinetic Herb-Drug Interactions of Glipizide with Andrographis paniculata (Burm. f.) and Andrographolide in Normal and Diabetic Rats by Validated HPLC Method. Molecules. 2022;27(20):6901. doi:10.3390/molecules27206901
How to cite this article:
Citation Formats:
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India