Antibacterial activity, cytotoxicity, and phytochemicals screenings of binahong (Anredera cordifolia (Ten.) steenis) leaf extract
Abstract
Streptococcus mutans can cause caries in human teeth. Currently, people are starting to turn to herbal plants, one of which is the binahong plant known to be used as an antibacterial. Herbal plants must have biocompatibility properties with the cell in the body. To determine the antibacterial effectiveness of the ethanol extract of binahong leaves against S. mutans and to determine its toxic effect on fibroblast cells. The antibacterial activity of binahong leaf extract was evaluated by the agar diffusion method (Kirby-Bauer). The method used for the cytotoxicity test in this study is the MTS Assay method. The phytochemical screening test of the sample ethanol extract of binahong leaves contained secondary metabolites of tannins, flavonoids, saponins, and triterpenoids. The results showed that binahong can be used as an antibacterial with an average inhibition zone diameter of 14.513mm at a concentration of 100%. There was no cytotoxicity effect on fibroblast cells in vitro. The IC50 result of 983.88g/mL is safe to use as a limit value. Binahong plant which contains active biological components has significant antibacterial activity against S. mutans and a very low toxic effect on fibroblast cells, so it can be used and developed as an ingredient of natural antibacterial.
Keywords: Antibacterial, Binahong leaf, Caries, Cytotoxicity, Streptococcus mutans
Introduction
The oral cavity is an integral part of the body, which is an ideal place for the growth of bacteria that have an important role in the body. However, if this environment is not balanced, then bacteria that were originally not pathogenic can become pathogenic and cause disease in the oral cavity [1, 2]. One of the diseases that can occur in the oral cavity is caries. Caries is a multifactorial infectious disease caused by three main factors, namely agents or microorganisms, host factors, substrates or diet, and coupled time factors [3]. In the occurrence of dental caries, microorganisms have a very important role. One of the pioneer microorganisms that can cause dental caries is Streptococcus mutans, which is the most cariogenic bacteria [4]. These bacteria will ferment sucrose into lactic acid which will cause the pH in the oral cavity to be low, thus causing a demineralization process [5].
To prevent caries, many antibacterial agents have been developed recently. However, many of these antibacterial agents can cause cell death in the oral mucosa which in turn can lead to a decrease in the body's defense mechanisms. Therefore, caries prevention agents need to be developed namely, agents that have an antibacterial effect but their effect on oral tissues must be evaluated so that they are safe for cells in oral tissues [1]. One of the dominant cells in connective tissue in the oral mucosa is fibroblasts which function to produce an extra matrix of connective tissue cells. Fibroblasts are also the largest cell component that composes dental pulp, periodontal ligament, and gingiva [6, 7]. Therefore, one of the efforts that can be developed is by utilizing medicinal plants that contain various biologically active components that can act as antibacterial, not antibacterial. toxic to cells, and can regenerate cells with minimal side effects [4].
The use of medicinal plants is growing rapidly among the people of Indonesia because these plants are efficacious for curing various kinds of diseases [8]. In Indonesia, the drug industry has succeeded in utilizing about 300 types of plants from around 9,600 types of plants that have the potential to be developed into medicinal plants [9]. One of the plants that can be developed into medicinal plants is the binahong leaf (Anredera cordifolia (Ten.) Steenis) [8]. Binahong is a medicinal plant from mainland China since the 14th century which is known by the original name dheng san chi, all parts of the plant can be used, starting from the roots (tubers), stems, and leaves. Binahong leaf was chosen in this study because this leaf is an herbal plant that contains several active compounds, including tannins, flavonoids, saponins, and triterpenoids, and is widely found in Southeast Asia and the tropical region of the United States [10, 11].
The active compound acts directly as an antibacterial against Streptococcus mutans by interfering with its function so that the growth of the bacteria that causes dental caries will be inhibited [12, 13]. Where the active compound derived from the binahong leaves must have good biocompatibility properties, both locally and systemically [14].
The purpose of this study was to determine the antibacterial effect of binahong leaf extract against S. mutans and to determine its toxic effect on fibroblast cells so that the use of binahong leaf extract in preventing caries would be safe for tissues in the oral cavity.
Materials and Methods
Binahong leaf ethanol extract (BLE) production
Binahong leaves used in this study were obtained from the Agricultural Technology Research and Development Installation Manoko, Lembang, West Java, Indonesia. The selected plants were planted at an altitude of 1.200 above sea level, with an age of about 6 months. Plant determination was carried out in the Botanical Division of the BRIN Biological Research Center, Cibinong, Bogor City, West Java, Indonesia. BLE was made by the maceration method using 70% ethanol solution and carried out a phytochemical test.
Making streptococcus mutans growing media
The growing media used in this study were Mueller Hinton Agar (MHA) (Himedia, M173) and Mueller Hinton Broth (MHB) (Himedia, M391).
BLE preparation for antibacterial test
The stock solution of BLE used was prepared by dissolving 3000mg of extract paste in 3 mL of 10% DMSO. Preparation of Working Solution (WS) Series: The stock dilution of the BLE was carried out using 10% DMSO to make a concentration series are 100%, 75%, 50%, 25%, 12.5%, 6.25%, and 3.125%.
Preparation of streptococcus mutans inoculum (ATCC 25175®TM)
The inoculum was obtained by inoculating Streptococcus mutans colonies that had been cultured for 24 hours in MHA medium into MHB medium. The turbidity of the solution was then adjusted to the turbidity of the standard McFarland 0.5.
Agar disk diffusion
Dip a sterile cotton swab into the bacterial suspension and wipe it on the surface of the MHA. Place a 6mm paper disc on the plate, and drop 10µL of each extract concentration, 0.1% Clindamycin, and 10% DMSO, doing 3 repetitions. Then incubate (Thermo IH3543) the agar plate at 37oC for 24 hours. Measure the diameter of the inhibition zone formed using a caliper (Modern, Sigma 6”).
According to Davis and Stout, the criteria for antibacterial strength based on the diameter of the inhibition zone are divided into several categories [15];
Weak: 5 mm or less.
Medium: 5-10 mm.
Strong: 10-20 mm.
Very Strong: more than 20 mm.
BALB/3T3 thawing cell procedure (ATCC®CCL-163)
Take the cell from the liquid nitrogen tank (-196oC) and a 3T3/BALB cell culture medium was prepared by mixing 10% FBS, 1% Antibiotic-Antimycotic, 1% Amphotericin B, 1% MEM Vitamins, 1% L-Glutamine, 0.2% Nanomycopulitine, 0.1% Gentamicin and DMEM High Glucose basal medium up to 100% of the total volume in a 50mL tube. Cells were centrifuged (MW260r) at 1600rpm for 5 minutes, and then the pellet was resuspended with 4mL of culture medium. The cell suspension was placed in flask T25 and was incubated (Thermo IH3543) at 5% CO2, at 37°C, and observed under an inverted microscope (Olympus CKX-41-F32FL) until the confluent was about 70-80%.
BALB/3T3 cell subculture procedure
The culture medium was removed and then rinsed with PBS. Add 2mL of Trypsin-EDTA, and then incubate for 3 minutes, CO2 5% at 37°C. Cells were centrifuged at 1600rpm for 5 minutes. Use 1 mL of culture medium to resuspend the pellets. then divided into 2 T25 flasks and incubated at 5% CO2, 37°C. Every 2-3 days, during cell treatment, the growth medium is replaced.
Preparation of ble sample solution for cytotoxicity test
The stock solution of BLE used was prepared by dissolving 50mg of extract paste in 1 mL of 100% DMSO. Preparation of WS Series: The stock dilution of the BLE was carried out using 10% DMSO to make a concentration series, which are 5.000µg/mL, 2500µg/mL, 1250µg/mL, 625µg/mL, 312.5µg/mL, 156.25µg/mL, 78.13µg/mL, and 39.06µg/mL.
Cytotoxicity test procedure
Hemocytometer (Neubauer) was used to count cells and cells were grown at a density of 5x103cells/well on a 96-well plate, then incubated (Thermo IH3543) for 24 hours at 37°C, 5%CO2. The sample was added to each well plate, then incubated for 24 hours at 37°C, 5% CO2, and added to each well plate 20µl of MTS, incubated for 3 hours at 37°C, 5% CO2. The absorbance was measured using spectrophotometry (Multiskan GO Thermo Scientific 51119300) and read at a wavelength of 490nm.
IC50 calculation
IC50 calculation in this study uses probit regression analysis using IBM SPSS Statistics version 26.
Statistics
The test to prove the antibacterial effectiveness of the ethanol extract of binahong leaves against Streptococcus mutans used the Kruskal Wallis non-parametric test, while to prove its toxic effect, the One Way Anova test was used with the Shapiro-Wilk normality test (p>0.05), which was then followed by Dunnett T3 Post Hoc. Test.
Results and Discussion
Determination test results
The results obtained from the determination test are:
Scientific Name: Anredera cordifolia (Ten.) Steenis
Local Name: Binahong
Tribe/Family: Basellaceae
Phytochemical screening test results
The results of the phytochemical screening test of the sample showed that the ethanolic extract of binahong leaves contained secondary metabolites of tannins, flavonoids, saponins, and triterpenoids.
|
Table 1. Results of Phytochemical Screening of Binahong Leaf Ethanol Extract |
|||
|
No |
Secondary Metabolites |
Test Method |
Test Results |
|
1 |
Tanin |
Reagent FeCl3 1% |
++ |
|
2 |
Flavonoid |
|
- + + |
|
3 |
Saponin |
Heated |
++ |
|
4 |
Triterpenoid |
Reagent-concentrated H2SO4+anhydrous CH3COOH |
+ |
(+): A little; (++): Medium; (+++): Many; (-): There isn't any
The antibacterial Effectiveness of BLE against Streptococcus mutans with the average (mean) and standard deviation (stdev) is presented in Table 2.
|
Table 2. Inhibitory Zone Diameter of BLE |
|||
|
Group |
Inhibition Zone Diameter |
Inhibition Zone Category (Davis-Stout) |
|
|
|
mean |
stdev |
|
|
Positive Control |
14.570 |
0.233 |
Strong |
|
Negative Control |
0.000 |
0.000 |
Weak |
|
BLE 3,125% |
0.000 |
0.000 |
Weak |
|
BLE 6,25% |
0.000 |
0.000 |
Weak |
|
BLE 12,5% |
7.133 |
0.252 |
Medium |
|
BLE 25% |
8.753 |
0.197 |
Medium |
|
BLE 50% |
9.500 |
0.737 |
Medium |
|
BLE 75% |
11.970 |
0.070 |
Strong |
|
BLE 100% |
14.513 |
0.096 |
Strong |
Antibacterial Effectiveness Test Results The antibacterial effectiveness test of the ethanol extract of binahong leaves (BLE) against Streptococcus mutans with the average (mean) is presented in Figure 1.
|
|
|
Figure 1. Average Inhibition Zone Diameter of Binahong Leaf Ethanol Extract (BLE) |
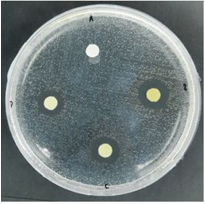
Based on Table 2 and Figure 1, it can be seen that the average diameter of the inhibition zone of the ethanol extract of binahong leaves in the positive control group was 14.57mm, and the average inhibitory power in the 100% BLE group was 14.513mm, the 75% BLE group was 11.970mm, the average of the BLE 50% group was 9.5mm, the average in the 25% BLE group was 8.753mm and the average in the 12.5% BLE group was 7.133mm. This indicates that the 100% BLE group is closest to the average diameter of the positive control group (Figure 2).
|
a) |
|
b) |
|
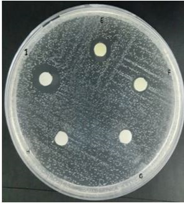
Figure 2. Observation of the zone of inhibition against Streptococcus mutans Description: A: Negative Control (DMSO 10%), B: BLE 100%, C: BLE 75%, D: BLE 50%, E: BLE 25%, F: BLE 12.5%, G: BLE 6.25%, H: BLE 3.125%, I: Clindamycin 0.1% |
From the results that have been obtained and processed statistically, it can be seen that the Chi-Square obtained is 25.451 > Chi-table 15.507, so it can be concluded that there are significant differences in antibacterial effectiveness between the concentration groups of binahong leaf ethanol extract. So, the higher the concentration of ethanol extract of binahong leaves, the higher the inhibitory power in inhibiting the growth of Streptococcus mutans bacteria.
Cytotoxicity test results
The results of measuring the viability of fibroblast cells that have been treated with BLE can be seen in Table 3.
|
Table 3. Cytotoxicity Test Results of BLE on BALB/3T3 cells |
||
|
Treatment |
Cell Viability (%) |
Cell Inhibition (%) |
|
Negative Control |
100.00 ± 1.21g |
0.00 ± 1.21a |
|
Positive Control DMSO 1% |
93.48 ± 1.32bcd |
6.52 ± 1.32de |
|
BLE 500µg/mL |
84.07 ± 0.73ab |
15.93 ± 0.93f |
|
BLE 250µg/Ml |
87.92 ± 0.87abc |
12.08 ± 0.87ef |
|
BLE 125µg/mL |
92.51 ± 0.14bc |
7.49 ± 0.14de |
|
BLE 62.5µg/mL |
94.91 ± 0.26de |
5.09 ± 0.26cd |
|
BLE 31.25µg/mL |
96.31 ± 0.29f |
3.69 ± 0.29b |
|
BLE 15.63µg/mL |
99.05 ± 0.32g |
0.95 ± 0.32a |
|
BLE 7.81µg/mL |
100.95 ± 0.74g |
-0.95 ± 0.74a |
|
BLE 3.91µg/mL |
106.84 ± 1.99g |
-6.84 ± 1.99a |
Data are presented in mean ±SD. Different superscript marks indicate a significant difference (p < 0.05) Dunnett T3 post hoc test.
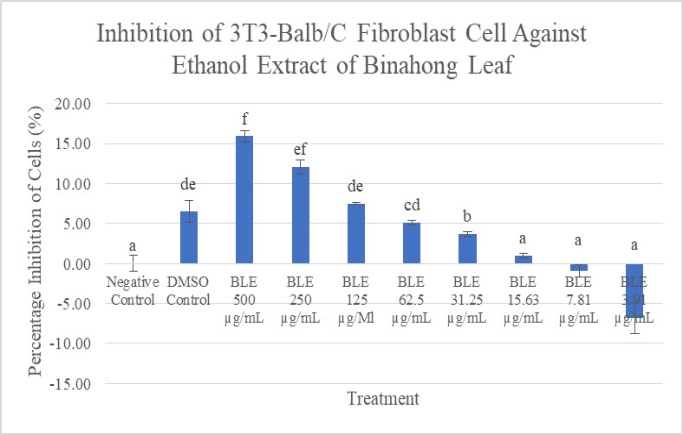
In this study, it can be seen in Figure 3, that the number of living cells is inversely proportional to the increase in concentration. The concentration group of 3.91g/mL had the highest number of living cells with a viability of 106.84%, indicating that this concentration could trigger the growth and proliferation of fibroblast cells. This is supported by the percentage of inhibition in Figure 4, the ethanol extract of binahong leaves at a concentration of 7.81g/mL was -6.84%.
|
|
|
Figure 3. Viability of BALB/3T3 cells after being given the Binahong Leaf Ethanol Extract with various concentrations. The data are presented in the mean ±SD. Different superscript signs (a, ab,b,c,d) showed a significant difference (p < 0.05) Dunnett T3 post hoc test |
|
|
|
Figure 4. Percentage of inhibition of BALB/3T3 cells after being given Binahong Leaf Ethanol Extract with various concentrations. Data are presented in mean ±SD. Signs of different superscripts (a, ab,b,c, cd,d) showed significant differences (p < 0.05) Dunnett T3 post hoc test |
The criteria for showing cytotoxicity can be seen in the classification according to ISO 10993-5: Biological Evaluation of Medical Devices, which states that if the relative cell viability for the extract concentration of a sample is less than 70%, then a substance or compound is considered to have toxic properties. So it can be concluded that the cell viability of the ethanol extract of binahong leaves which is still above 90% is considered a safe dose [16].
Calculation of IC50 value
The IC50 value is the potential of a substance in inhibiting biological functions up to 50%, the value is taken from the results of probit statistics with a probability of 0.5 (which means 50%), which means that the ethanolic extract of binahong leaves can inhibit 3T3-Balb/C fibroblast cells by 50% at a concentration 983.88g/mL.
The results of laboratory experimental research on the antibacterial effectiveness of the ethanolic extract of binahong leaves with various concentrations showed that the ethanolic extract of binahong leaves had an antibacterial effect which was indicated by its effectiveness in inhibiting the growth of Streptococcus mutans bacteria as presented in Table 1. Based on the results of qualitative phytochemical tests, ethanol extracts of Binahong leaves contain active compounds such as tannins, saponins, flavonoids, and triterpenoids. It is the content of the active compounds in the binahong leaves that can play an important role as an antibacterial and can inhibit the growth of Streptococcus mutans bacteria which is the bacteria that causes dental caries [12, 13].
The binahong plant used in this study was found to contain different amounts of flavonoid, triterpenoid, saponin, and tannin compounds. This can happen because it is influenced by internal factors and external factors, including biotic and abiotic conditions. Internal factors include genetic factors and physiological factors such as the level of development of a plant. Meanwhile, external factors are influenced by abiotic environmental factors, namely climate and biotic environment such as interactions with other organisms, and are influenced by geographical conditions of environmental stress that induce secondary metabolite biosynthesis or defensive responses [17, 18]. Climate change such as temperature, drought, CO2, light, salinity, UV light, ozone, and other factors can cause changes in plant metabolism which will affect the secondary metabolites they contain. Therefore it can be said that each plant species requires special environmental conditions for its growth [19, 20].
Tannin compounds are one type of compound that is included in the polyphenol group and has properties that are soluble in water and organic solvents that are often found in plants. This compound has a phenol group that is antiseptic and can form complex compounds with proteins in bacteria through hydrogen bonds. The formation of hydrogen bonds between tannins and proteins is what allows bacterial cell proteins to be denatured so that bacterial metabolism is disrupted. Tannins also have toxic properties, so these compounds can damage bacterial cell membranes and are thought to cause shrinkage of the cell wall or bacterial cell membrane, thereby disrupting the permeability of the cell itself, and ultimately the cell cannot carry out its activities [21, 22].
As an antibacterial, saponin compounds will be associated with lipopolysaccharides in the bacterial cell wall. This will decrease the surface tension and increase the permeability of the cell wall so that antibacterial substances can easily enter the cell. This will cause an interaction that causes the cell wall to undergo lysis and result in bacterial death [23].
Antibacterial effects can also occur due to the presence of flavonoid compounds, where these compounds will inhibit cytoplasmic membrane function, nucleic acid synthesis, and the energy metabolism of bacteria [24]. Flavonoids can form complexes with bacterial cell walls, and extracellular proteins, and can damage bacterial cell membranes because flavonoid is lipophilic [25]. Flavonoids in some plants are known to have antibacterial properties by releasing transduction energy to the bacterial cytoplasmic membrane and inhibiting bacterial motility, that the hydroxyl group in the flavonoid structure causes changes in organic components and nutrient transport which can eventually lead to the toxic effect on bacteria [23].
Triterpenoid as an antibacterial has a mechanical action is on the outer membrane of the bacterial cell wall by reacting with the porin (transmembrane protein), forming a strong polymer bond, resulting in the destruction of the porin. Damage to the porin will reduce the permeability of the bacterial cell wall which is the entrance and exit of the compound and cause the bacterial cell to lack nutrients so that bacterial growth is inhibited or dead [26].
In addition to knowing the role of the biologically active content as an antibacterial of this medicinal plant, the toxic properties of cells and tissues also need to be considered before this plant will be used in humans [27]. Therefore, a cytotoxicity test on fibroblast cells is needed to directly determine the toxic effect of a substance on tissue culture and observe cell growth, reproduction, and morphological effects. The principle of this test is to measure the ability of cell viability in a material [28, 29].
The mechanism of cytotoxicity of a substance to cell culture occurs through the destruction of cell membranes, prevention of protein synthesis, binding to cell receptors, inhibiting polydeoxynucleotide elongation, and enzymatic reactions. The parameter of this test is the value of inhibition concentration (IC50). This value indicates the concentration of extracts or ingredients that can inhibit cell proliferation by 50% in the total population [29].
The results of this study indicate that the average dose used in this test is still within a safe dose, based on the classification according to ISO 10993-5: Biological Evaluation of Medical Devices, which states that the relative cell viability for the extract concentration of a sample is more than 70% then a substance or compound is considered not to have toxic properties [16]. The determination of IC50 is a measure of drug efficacy that indicates the amount of concentration of a chemotherapeutic agent required to inhibit biological processes up to 50%. IC50 obtained in this study can be used as a reference for further research on the potency of the extract. binahong leaf ethanol.
In this study, the IC50 value was 983.88 g/mL, thus the IC50 result of the ethanolic extract of binahong leaves was safe to use as a limit value because it did not interfere with cell proliferation. If the dose used exceeds the IC50 value, it can disrupt the cell proliferation phase, because fibroblast migration does not occur or even fibroblasts die. So it can be said that the concentration of ethanol extract from binahong leaves used in this study was relatively safe because the cell viability value was still above 90%.
In addition to having an antimicrobial effect, the active compound content of binahong leaves can also play a role in the tissue healing process because when used in certain doses it is not toxic and has a role as an antioxidant, anti-inflammatory, and antibacterial so that it can be used in the wound healing process [30]. Saponin compounds can increase monocyte proliferation thereby helping to increase the number of macrophages that will secrete growth factor TGF-β1 which will play a role in various phases of wound healing [31, 32].
The content of tannins and triterpenoids which act as antibacterial also acts as an astringent in wounds and helps in the differentiation process of myofibroblasts [33, 34]. The content of flavonoids can inhibit the formation of prostaglandins formed by arachidonic acid and other inflammatory mediators such as histamine and serotonin. The high content of flavonoids in binahong leaves plays a role in the cell proliferation phase during the wound healing process by increasing the process of mitogenesis, cell interactions, and molecular adhesion [31].
Conclusion
Binahong plant (Anredera cordifolia (Ten.) Steenis) which contains active biological components of tannins, flavonoids, saponins, and triterpenoids has significant antibacterial activity against S. mutans and a very low toxic effect on fibroblast cells, so it can be used and developed as an ingredient. natural antibacterial and helps in the process of repair and regeneration.
Acknowledgments: The author would like to thank Maranatha Christian University, Indonesia
Conflict of interest: None
Financial support: The present study was supported by Maranatha Christian University Indonesia.
Ethics statement: None
References
- Bowen J, Cole C, McGlennen R. Comparison of Antimicrobial and Wound Healing Agents on Oral Fibroblast Viability and In-vivo Bacterial Load. Dentistry. 2015;5(6):1-6.
- Meskhi R, Montazeri EA, Khoshroo S, Kazemi M, Saki M. The Effect of Pascal and Preventa Fluoride Varnishes on The Salivary Streptococcus mutans Count (In Vivo). Ann Dent Spec. 2018;6(2):124-7.
- Ramayanti S, Purnakarya I. Peran makanan terhadap kejadian karies gigi. J Kesehat Masy. 2013;7(2):89-93.
- Sateriale D, Imperatore R, Colicchio R, Pagliuca C, Varricchio E, Volpe GM, et al. Phytocompounds vs. Dental Plaque Bacteria: In vitro Effects of Myrtle and Pomegranate Polyphenolic Extracts Against Single-Species and Multispecies Oral Biofilms. Front Microbiol. 2020;11:1-15.
- Nuraini A, Setyawan S, Susilawati S. Gambaran skor karies menurut status kehamilan di puskesmas bayat kabupaten klaten. J Kesehat Masy. 2018;6(5):253-8.
- Emilda Y, Budipramana E, Kuntari S. Uji toksisitas ekstrak bawang putih (Allium sativum) terhadap kultur sel fibroblast. Dent J. 2014;47(4):215.
- Wangko S, Karundeng R. Komponen sel jaringan ikat. J Biomedik. 2014;6(3):1-7.
- Sihotang T, Jayawardhita A, Berata I. Efektivitas pemberian gel ekstrak daun binahong terhadap kepadatan kolagen pada penyembuhan luka insisi mencit diabetes. Indones Med Veterinus. 2019;8(4):456-63.
- Haras MS, Assa JR, Langi T. Tingkat penerimaan konsumen terhadap teh daun binahong (Anredera cordifolia (Ten.) Steenis) pada variasi suhu dan waktu penyeduhan. InCOCOS 2017 Aug 1 (Vol. 1, No. 6).
- Larissa U, Wulan A, Prabowo A. Pengaruh binahong terhadap luka bakar derajat kedua. J Major. 2017;7(1):130-4.
- Riyandi F, Proklamasiningsih E, Rochmatino R. Pengaruh pemberian asam humat pada media tanam terhadap pertumbuhan dan kandungan polifenol daun binahong (Anredera cordifolia). J Ilmu Biol Univ Jenderal Soedirman. 2020;2(2):243.
- Istiqomah N, Santoso B. Pengaruh berkumur larutan daun binahong terhadap ph saliva pada ibu hamil desa Babadan kabupaten Semarang. J Kebidanan. 2015;4(9):31-6.
- Warokka K, Wuisan J, Juliatri. Uji konsentrasi hambat minimum (khm) ekstrak daun binahong (Anredera cordifolia (Ten.) Steenis) sebagai antibakteri terhadap pertumbuhan Streptococcus mutans. e-GIGI. 2016;4(2):15-27.
- Lestari D, Kartika R, Marliana E. Uji brine shrimp lethality test (BSLT) umbi bawang tiwai (Eleutherine bulbosa (Mill.) Urb) dan uji toksisitas akut fraksi aktif. J Ris Kefarmasian Indones. 2019;1(1):1-10.
- Andries J, Gunawan P, Supit A. Uji efek anti bakteri ekstrak bunga cengkeh terhadap bakteri Streptococcus mutans secara in vitro. e-GIGI. 2014;2(2):2-8.
- Standardization IO. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. 2009. ISO: Geneva, Switzerland.
- Darmawan, Yusuf M, Syahruddin I. Pengaruh berbagai media tanam terhadap pertumbuhan bibit tanaman kakao (Theobroma cacao L.). J Agroplantae. 2015;4(1):13-8.
- Uddin M. Environmental factors on secondary metabolism of medicinal plants. Acta Sci Pharm Sci. 2019;3(8):34-46.
- Pant P, Pandey S, Dall'Acqua S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem Biodivers. 2021;18(11):1-14.
- Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020;148:80-9.
- Bestriandita D, Widodo E. Analisis Perbandingan Efektivitas Iklan Menggunakan EPIC Model Terhadap Mahasiswa UII Yogyakarta. InProsiding SI MaNIs (Seminar Nasional Integrasi Matematika Dan Nilai-Nilai Islami) 2017 Jul 31 (Vol. 1, No. 1, pp. 214-220).
- Utomo S. Uji aktivitas antibakteri senyawa c-4-metoksifenil kaliks resorsinarena termodifikasi hexadecyltrimethylammonium-bromide terhadap bakteri Staphylococcus aureus dan Escherichia coli. J Kim dan Pendidik Kim. 2018;3(3):201-9.
- Manik D, Hertiani T, Anshory H. Analisis korelasi antara kadar flavonoid dengan aktivitas antibakteri ekstrak etanol dan fraksi-fraksi daun kersen (Muntingia calabura L.) terhadap Staphylococcus aureus. Khazanah. 2014;6(2):1-11.
- Pendit P, Zubaidah E, Sriherfyna F. Karakteristik fisik-kimia dan aktivitas antibakteri ekstrak daun belimbing wuluh (Averrhoa bilimbi L.). J Pangan dan Agroindustri. 2016;4(1):400-9.
- Dwicahyani T, Sumardianto, Rianingsih L. Uji bioaktivitas ekstrak teripang keling holothuria atra sebagai antibakteri Staphylococcus aureus dan Escherichia coli bioactivity. J Pengolahahn dan Biotek. 2018;7(1):15-24.
- Halimah H, Suci DM, Wijayanti I. Studi Potensi Penggunaan Daun Mengkudu (Morinda citrifolia L.) sebagai Bahan Antibakteri Escherichia coli dan Salmonella typhimurium. J Ilmu Pertan Indones. 2019;24(1):58-64.
- Ribeiro M, Malheiro J, Grenho L, Fernandes MH, Simões M. Cytotoxicity and antimicrobial action of selected phytochemicals against planktonic and sessile Streptococcus mutans. PeerJ. 2018;6:e4872.
- Restiani Q, Rukmo M, Juniarti D. Uji sitotoksisitas ekstrak daun mimba (Azadirachta indica) terhadap sel fibroblas BHK21. Conserv Dent J. 2017;7(1):48-52.
- Aslantürk ÖS. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. Genotoxicity - A Predict Risk to Our Actual World. 2018;38(10):4-5. doi:10.5772/intechopen.71923
- Kurniawan B, Aryana W. Binahong (Cassia alata L.) as an inhibitor of escherichia coli growth. Majority. 2015;4(4):100-4.
- Ardiana T, Kusuma AR, Firdausy MD. Efektivitas pemberian gel binahong (Anredera cordifolia) 5% terhadap jumlah sel fibroblast pada soket pasca pencabutan gigi marmut (Cavia cobaya). ODONTO Dent J. 2015;2(1):64-70.
- Kaur G, Utami N, Usman H. Effect of topical application of binahong (Anredera cordifolia (Ten.) Steenis) leaf paste in the wound healing process in mice. Althea Med J. 2014;2(5):6-11.
- Anggriawan M, Yuliet, Khaerati K. Pengaruh Pemberian Topikal Ekstrak Etanol Daun Pecut Kuda ( Stachytarpheta jamaicensis ( L .) Vahl) terhadap Penyembuhan Luka Bakar Derajat II pada Punggung Kelinci. Biocelebes. 2018;12(2):47-8.
- Noer S, Pratiwi RD, Gresinta E, Biologi P, Teknik F. Penetapan Kadar Senyawa Fitokimia (Tanin, Saponin Dan Flavonoid Sebagai Kuersetin) Pada Ekstrak Daun Inggu (Ruta angustifolia L.). J Eksakta. 2018;18(1):19-29.
How to cite this article:
Citation Formats:
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India