The impact of increased body weight on left ventricular diastolic function
Nebaa Raheem Wahab1, Mohammed N. Al-dujaili2*, Hassan Ali Hassan AL-Khirsani3, Abdullah Abass Mohammed4
1 Rrsearcher in Al-Sader teaching hospital, Najaf, Iraq. 2 Faculty of Medicine, University of Kufa, Iraq. 3 Consultant in Al-Sader teaching hospital, Najaf, Iraq. 4 Assist lectural in ministry of youth and sport, Iraq.
ABSTRACT
Background: Diastolic dysfunction is a problem, due to an impairment in the filling properties of the left ventricle (LV), indicating future heart failure. In obese patients, this problem is a prevalent cause of heart failure in which the systolic function is maintained. Aim of the study: This study was conducted to evaluate the independent effect of increased body weight with different degrees on LV diastolic function. Patients and methods: A retrospective cohort study was done at Al-Sader Medical City in Al Najaf City, Echocardiographic Department during the period from March 2018 to March 2019. 120 patients (age: 61.59 ± 13.9 years) were chosen and LV diastolic function, body mass index (BMI), and waist circumference (WC) were assessed. The patients were divided into 3 groups based on their body mass index (kg/m2): [obese < (29.9); overweight, (25–29.9); normal, (18.5–24.9)]. Peaks late (A) and early (E) transmitral velocities and also peak early diastolic mitral annulus velocity (E′) were measured. Data analysis and management were done by using the SPSS software version 24, 2013. Results: Diastolic dysfunction in the obese/overweight groups was considerably higher than the group with normal BMI. By analyzing the multivariate regression, BMI, and other characteristics, the independent and direct effect of BMI on diastolic function was demonstrated [OR: 2.65; confidence interval (CI): 1.46–5.76; P = 0.007]. Conclusion: Obesity and overweight have a negative independent effect on diastolic function.
Keywords: body weight, left ventricular, Diastolic, Body weight, Obesity.
Introduction
Obesity
Currently, Obesity is a major cause of preventable death and it increases the risk of dyslipidemia, hypertension, type 2 diabetes mellitus, cardiovascular disease (CVD), stroke, cancer (breast, colon, and endometrial), osteoarthritis, obstructive sleep apnea, and other diseases. These risks increase with rising BMI. Obesity is also associated with reduced quality of life, impaired physical functioning, and increased health care costs. Central adiposity (waist circumference >102 cm [40 in] in men and >88 cm [35 in] in women) increases the cardiovascular risk independent of BMI [1].
Obesity increases steadily throughout the world and becomes a leading health problem because it is associated with mortality, morbidity, and CVD [2-4].
DIASTOLE
- Physiology of Diastole
Diastole is a Greek word meaning (“expansion”), defined as the time between the aortic valve closure and mitral inflow termination. On the ECG diastole is the time between the end of QRS complex and T-wave. A normal diastolic function is as the left ventricle ability to guarantee enough stroke volume at the low-pressure state and its capacity of receiving a filling volume [5]. diastole is divided into 4 phases:
- Isovolumetric Relaxation.
- Rapid ventricular Filling.
- Diastasis.
- Atrial Contraction.
- Isovolumetric relaxation: A period between the aortic closure and mitral opening, when LV decreases its tension without lengthening so the ventricular volume remains unaltered.
- LV rapid filling: begins with falling LV pressure below the left atrial pressure and opening the mitral valve. In this phase, the blood is accelerated and reaches the maximal extent, which has a direct relation to the atrioventricular pressure. When this gradient is finished, the process ends. In this period, there is a complex interaction between myocardium compliance and LV suction (active relaxation).
- Diastasis: This happens when LV filling is maintained by the blood coming from the pulmonary vein and its amount depends on LV pressure. LV and LA pressures are almost equal in diastasis.
- Atrial systole: This corresponds to LA contraction and ends when the mitral is closed. This period is related to the compliance of LV and slightly to the atrial force, the resistance of pericardium, and atrioventricular synchronicity (electrocardiogram-derived PR interval) [6].
Echocardiography is more accurate than ECG in determining the duration of diastole, and to distinguish between the different phases of diastole. Both PW Doppler of mitral inflow and LVOT flow are used to measure diastolic time intervals as shown in (figure 1). Tissue Doppler at the mitral annulus is also used to assess the various phases of diastole:
- IVRT – The time between the ending of atrioventricular flow and the beginning of E-wave.
- Rapid LV filling - Duration of the E-wave.
- Diastasis: Time between E- and A-wave.
- Atrial contraction: Duration of the A-wave [7, 8].
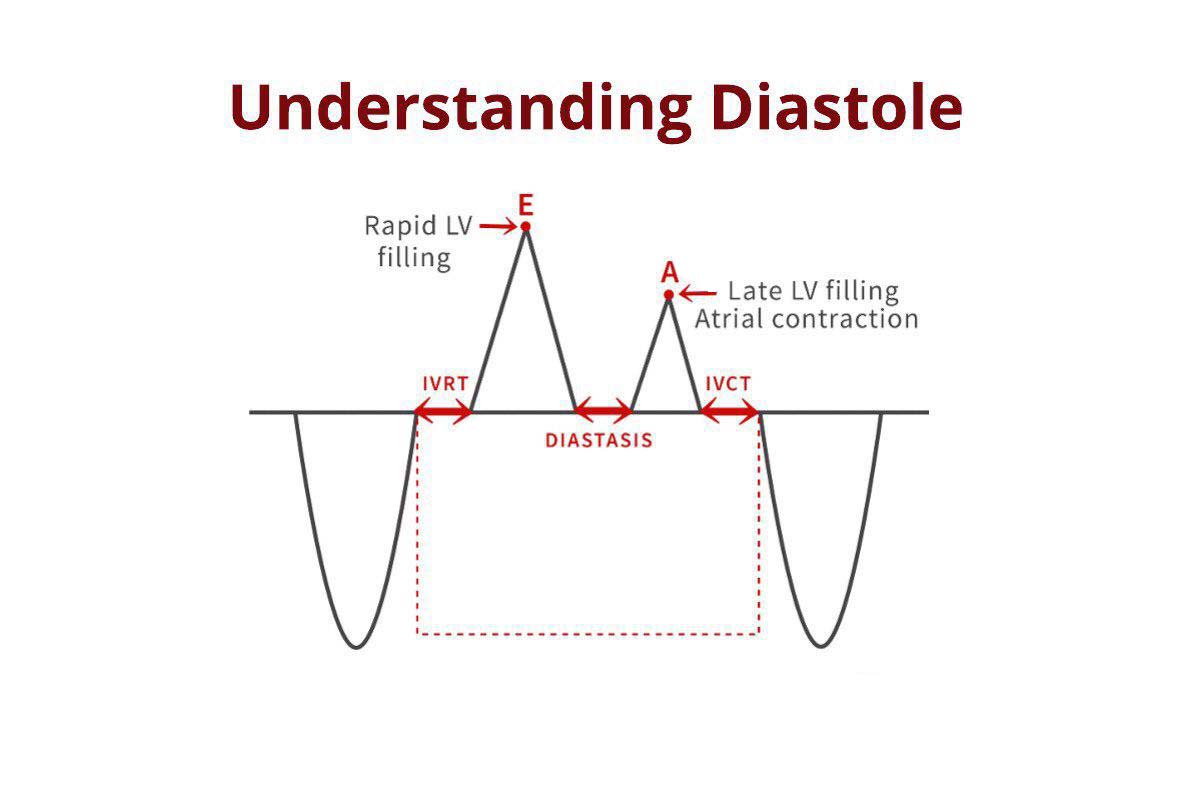
Figure 1. phases of diastole
- Diastolic dysfunction:
LVDD is a condition that shows the impairment in the filling properties of the left ventricle, which is a predictor of heart failure in the future [9-12].
- Pathology of diastolic dysfunction:
Various factors can cause diastolic dysfunction such as extrinsic factors, myocardial compliance decrease, and abnormal relaxation.
Relaxation: depends on the normal function of the ventricle and is disturbed when the systolic function is impaired, for example in diastolic function abnormalities, myocardial ischemia, and cardiomyopathy.
Compliance: This is a way of determining the ventricular stiffness and describes the ventricle ability to expand during diastole [5]. 3 factors can reduce the ventricle compliance:
- A thickened myocardial wall (left ventricular hypertrophy).
- Myocardial fibrosis (left ventricular hypertrophy and aging).
- Myocardial infiltration (infiltrative cardiomyopathy including hemochromatosis and amyloidosis).
“Extrinsic factors” are factors in which the myocardium itself is not the reason for the filling abnormality. These factors include pericardial disease, like pericardial effusion and constrictive pericarditis, in which the pericardial sac is stiff or intrapericardial pressure is increased.
- The overload of right ventricular pressure, as in pulmonary embolism or hypertension, in which intrapericardial pressure increases due to the transferring of augmented end-diastolic pressure (EDP) and volume (EDV) of the right ventricle to the left ventricle and leads to the impairment of left ventricular filling.
- Compression by pulmonary or mediastinal forces, as in the tumors and in mediastinal or interpericardial masses that compress the heart. Diastolic dysfunction usually is a multi-factor condition [13].
Doppler-Echocardiographic Diagnosis of LVDD:
LVDD may even be asymptomatic and identified occasionally during a Tissue Doppler-echocardiographic examination. This is important as a diagnostic tool because of its high feasibility of transmitral Doppler indexes of diastolic function (E/A ratio, DT, and IVRT), and is satisfactory for serial assessments over the time.
By using this approach, normal diastole can be recognized and also the progression of LVDD can be classified. Diastolic dysfunction was defined as 1) E/A <0.7 (impaired relaxation, grade I); or 2) E/A >0.7 and <1.5 and E/E’>8 cm/s (pseudonormalized pattern, grade II); or 3) E/A >1.5 and E/E’ >8 cm/s (restrictive pattern, grade III) [14, 15].
Pathophysiology of obesity-related LVDD
Obesity can be a predictor of incident heart failure in the general population, and evidence suggests that overweight status also increases heart failure risk, which is intermediate between that of lean and obese persons [16, 17]. Increasing the body size and being exposed to CVD risk factors including diabetes mellitus, hypertension, and hyperlipidemia, have direct effects on cardiac function and structure. The excessive body fat increases in both pre- and afterload because of an increase in peripheral resistance, chronic volume overload, and hyperdynamic circulation [18, 19].
Moreover, increased adiposity leads to the enhanced effect of blood pressure on the mass growth of LV [20]. LVDD might, therefore, be a pathophysiological link between the body weight gain and the onset of heart failure in the future. Cardiac structural changes and cardiovascular risk factors associated with overweight/obesity are also the main determinants of the diastolic function of LV [21, 22].
Aim of the study
This study was done to assess the independent effect of different degrees of increased body weight on LV diastolic function.
Materials and Methods
Study design:
A cohort retrospective study was conducted at the Cardiology Centre, Echocardiography Unit in AL-Sader medical city during the period of March 2018 to March 2019.
Ethical Approval:
The study was approved by the scientific council of Arab board for health specialization and informed consent was obtained verbally from all study participants.
We evaluated 120 participants who referred to the outpatient echocardiographic department and had medical record filled including demographic data (clinical status, gender, and age) were obtained via physical examination and detailed history-taking. Clinical conditions and risk factors were identified based on the self-report of the patients’ history, WC, blood pressure, heart rate, and BMI, with a resting electrocardiogram tracing for each participant. BMI was calculated as weight (kg) divided by height squared (m2). The overweight was defined as a BMI between 25.0 and 29.9 kg/m2, and obesity as 30 kg/m2 and above, based on the standard definition [23].
The participants were divided into normal weight (BMI <25) with 60 participants and elevated BMI with 60 participants, then the second group was subdivided into overweight and obese with 37 and 23 participants, respectively. Their gender and age were matched.
Inclusion criteria
If the patients fulfilled the following criteria, they were eligible for inclusion: sinus rhythm approved informed consent, age <65 years, and did not have exclusion criteria
Exclusion criteria
- Age >65 or <18 years.
- IHD
- segmental wall motion abnormalities.
- congenital heart disease.
- valvular heart disease.
- Structural heart disease.
- Hypertension.
- diabetes.
- HF
- restrictive pericarditis.
- Rate abnormality (bundle branch block, pacemaker dependency, an atrioventricular block of any degree, and a sinus rate of ≥120 bpm at the time of examination).
Echocardiographic assessment.
Transthoracic echocardiography was conducted using a commercially available system (Vivid E 9) by a trained physician following a standardized protocol. The linear dimensions of LV were measured from a parasternal long-axis view according to the American Society of Echocardiography recommendations [16]. The relative wall thickness of LV was calculated as follows: (2* posterior wall thickness divided by end-diastolic diameter) [19]. The LV ejection fraction was calculated by biplane modified Simpson’s rule. For the assessment of left ventricular diastolic function [13] from an apical 4-chamber view, the transmitral flow was sampled by pulsed-wave Doppler at the level of mitral leaflet tips. Peak velocities of the early (E) and late (A) phases of the mitral inflow were measured, and their ratio (E/A) was calculated. Myocardial velocities of LV were analyzed by Tissue Doppler Imaging (TDI). Pulsed TDI sample volume was placed at the level of the septal and lateral mitral valve annulus, and the peak early diastolic velocities (E’) were measured [20]. The E/E’ ratio was calculated as an index of the filling pressures of LV [21]. Diastolic dysfunction was defined as 1) E/A <0.8, DT >200 ms , IVRT >100 (impaired relaxation, grade I); or 2) E/A >0.8 and <1.5 and E/E’>8 cm/s DT 160-200 ms, IVRT (70-100 ms) (pseudonormalized pattern, grade II); or 3) E/A >1.5 and E/E’ >8 cm/s DT <160 ms ,IVRT <60 ms (restrictive pattern, grade III) [22, 24-27].
Results
Statistical Analysis
SPSS Software version 23.0 was used for statistical analysis. Categorical variables are shown as number and percentage, and continuous variables are presented as mean ± standard deviation. The comparison of study groups was done by using the chi-square test for categorical data and Student's t-test for continuous data. P-value of <0.05 was considered statistically significant.
Clinical characteristics and study subjects
Out of 120 studied subjects, (mean age of 43.85± 13.27 years, minimum 23, maximum 63) were eligible; 60 subjects had a normal BMI (group 1), 60 had abnormal BMI (overweight/obesity), 37 were overweight (group 2), and 23 were obese (group 3). Table 1 shows the gender and age distribution among the studied groups: there was not any significant difference between the groups regarding gender and age.
|
Table 1. Gender and age distribution of the studied groups |
||||||||||
|
P-value |
BMI |
Variables |
||||||||
|
Total NO. |
obese(n=23) |
overweight(n=37) |
normal (n=60) |
|
||||||
|
|
% |
NO |
% |
NO. |
% |
NO. |
||||
|
0.717 |
21 |
12.00% |
3 |
22.90% |
8 |
16.70% |
10 |
≤ 30 |
age(year) |
|
|
30 |
34.78% |
8 |
18.91% |
7 |
25.00% |
15 |
31- 40 |
|||
|
32 |
26.08% |
6 |
27.02% |
10 |
26.70% |
16 |
41- 50 |
|||
|
37 |
24.00% |
6 |
34.30% |
12 |
31,7% |
19 |
> 50 |
|||
|
44.91±10.24 |
43.56±14.57 |
43.62 ±10.80 |
mean ± SD |
|||||||
|
1.00 |
51 |
39.13% |
9 |
40.54% |
15 |
45.00% |
27 |
Male |
Gender |
|
|
69 |
60.86% |
14 |
59.45% |
22 |
55.00% |
33 |
female |
|||
Abnormal WC was encountered in 9 patients (15%) in group 1, 21 patients (56.7%) in group 2, and 23 patients (100%) in group 3. Out of the 9 patients with normal weight obesity (group 1), 2 had LVDD (1 pseudonormal pattern and 1 impaired relaxation,). Table 2 shows the clinical characteristics of each group of participants expressed as mean and standard deviation.
|
Table 2. Clinical characteristics of the studied groups |
||||
|
obese total(n=60) |
BMI |
clinical |
||
|
obese(n=23) |
overweight(n=37) |
normal(n=60) |
||
|
mean ± SD |
mean ± SD |
mean ± SD |
mean ± SD |
parameters |
|
44.08±13 |
44.91±10.24 |
43.56±14.57 |
43.62 ±10.80 |
Age |
|
85.21±12.6 |
95.65±10.54 |
78.72±8.92 |
67.59 ± 5.58 |
weight |
|
1.68±0.06 |
1.67±0.06 |
1.68±0.05 |
1.69± 0.05 |
height |
|
29.92±4.04 |
33.9±3.52 |
27.44±1.66 |
23.36± 1.45 |
B.M.I. |
|
1.95±0.18 |
2.04±0.13 |
1.89±0.19 |
1.77± 0.09 |
BSA |
|
100.66±17.07 |
108.13±9.42 |
96.01±19.12 |
89.85± 11.6 |
waist circum. |
Values of LV end-diastolic dimension and PWT were significantly higher in obese/overweight groups in comparison to the normal-weight participants (p-value=0.026±0.004), respectively. The E/A ratio was significantly lower in the participants with overweight in comparison to the normal-weight participants (p=0.036). The E/E' ratio was significantly higher in obese and overweight participants in comparison to the normal-weight persons (both p=0.001). Deceleration time (DT) was significantly and progressively higher in obese and overweight patients in comparison to the normal-weight persons (p-value=0,029)
There is no significant difference in EF %, IVRT, RWT, and LVESD among the studied groups (p-value>0.05). Echocardiographic results are shown in Table 3.
|
Table 3. Comparison of mean of echocardiographic parameters among the studied groups |
|||||
|
P-value |
BMI |
Echo parameters |
|||
|
total(n=60) |
obese( n=23) |
overweight(n=37) |
normal(n=60) |
||
|
mean ± SD |
mean ± SD |
mean ± SD |
mean ± SD |
||
|
0.076 |
62±3.61 |
62.73±4.35 |
61.54±3.04 |
62.51±3.65 |
E.F.% |
|
0.57 |
3.15±0.44 |
3.17±0.39 |
3.14±0.47 |
3.21±0.51 |
LVESD |
|
0.026* |
4.4±0.54 |
4.61±0.46 |
4.32±0.56 |
4.29±0.57 |
LVEDD |
|
0.004* |
0.92±0.11 |
0.95±0.12 |
0.9±0.09 |
0.78±0.14 |
PWT |
|
0.47 |
1.17±5.88 |
0.41±0.04 |
1.64±7.49 |
0.36±0.06 |
RWT |
|
0.029* |
207.36±37.1 |
209.82±47.28 |
205.83±29.72 |
197±27.8 |
D.T. |
|
0.071 |
87.85±12.4 |
89.82±15.42 |
86.62±10.14 |
82.8±14.3 |
IVRT |
|
0.036* |
0.93±046 |
0.87±0.63 |
0.95±0.31 |
1.09±0.27 |
E \ A |
|
0.001* |
8.24±2.52 |
8.9±3.08 |
7.83±2.03 |
6.92±1.06 |
E \ E' |
Out of 120 studied participants, LVDD was encountered in 32 patients (26.66%), 9 (15%) patients from the normal-weight group, 12 (32.4%) from the overweight group, and 11 (47.8%) from the obese group (P-value=0.007) as shown in (Table 4).
|
Table 4. LVDD distribution among the studied groups |
||||
|
P-value |
obese( n=23) |
overweight(n=37) |
normal(n=60) |
|
|
0.007* |
12(52.2%) |
25(67.5%) |
51 (85%) |
Normal |
|
|
11(47.8% ) |
12(32.4%) |
9 (15%) |
DD |
Data are expressed as percentages and counts.
Impaired relaxation was encountered in 7 (11.66), 5 (13.51%), and 4 (17.39%) patients from groups 1, 2, and 3, respectively. A pseudonormal pattern was encountered in 2 (3.33 %), 6 (16.21%), and 5 (21.73%) patients from groups 1, 2, and 3, respectively. The restrictive pattern was encountered in none (0%), one (2.7%), and two (8.69%) patients from groups 1, 2, and 3, respectively as shown in (Table 5).
|
Table 5. LVDD grades distribution among studied groups |
||||
|
total |
obese(n=23) |
overweight(n=37) |
normal(n=60) |
LVDD |
|
16(13%) |
4(17.39%) |
5(13.51%) |
7 (11.66) |
G1 |
|
13(10%) |
5 (21.73%) |
6 (16.21%) |
2(3.33 %) |
G2 |
|
3(2,5%) |
2 (8.69%) |
1 (2.7%) |
none (0%) |
G3 |
Data are expressed as percentages and counts
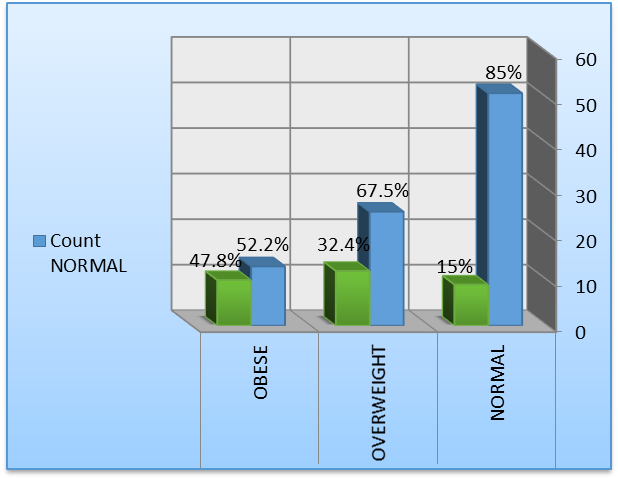
Figure 1: LVDD in the studied groups
For identifying factors with independent impact on LVDD, multivariate regression was analyzed and showed that the increased age and BMI had an independent and direct effect on diastolic dysfunction [OR: 2.65; confidence interval (CI): 1.46–5.76; P = 0.007] (Table 6).
|
Table 6. Multivariate regression analysis |
||||
|
P value |
CI |
OR |
variables |
|
|
|
Upper limit |
Lower limit |
|
|
|
0.007 |
5.76 |
1.46 |
2.65 |
BMI |
|
<0.0001 |
1.14 |
1.02 |
1.09 |
Age |
Discussion
In this study, we showed that increasing age and abnormal BMI are independent determinants of LVDD. The relationship between diastolic dysfunction and BMI parameters is continuous and independent of cardiovascular risk factors. Moreover, the severity and prevalence of LVDD were progressively higher from group 1 to group 3. Similarly according to the recent studies conducted by Kossaify., Kishi et al., C,il, et al., and Russo et al. [28-31] raising an individual's BMI predisposes him/her to LVDD, with a particular highlight on the TDI’s role in assessing the diastolic function in obesity [29, 32].
In this study, BSA increasing had a direct correlation with LVDD (p=0.005) and BMI (p <0.0001). Also, abnormal WC had a direct correlation with LVDD (P=0.003) and BMI (P<0.0001). Nevertheless, BSA and WC were not independent determinants of LVDD, and this disagreed with previously reported studies by Libhaber CD et al. and others [33-35]. Normal weight obesity (abnormal WC, normal BMI) was observed in 9 subjects, and only 2 of them had LVDD; Such a small number can be due to the insufficiency of the statistical power in the demonstration of an independent effect of normal weight obesity on LVDD. Moreover, fat distribution and concentration were not assessed by using markers like waist-to-hip ratio or the percentage of body fat.
There was not any significant correlation between LVDD and gender specification, which was similarly found in other studies [28-31].
By using TDI parameters diastolic abnormalities could be detected in the participants who would be classified as normal by Doppler flow analysis alone [26]. Furthermore, E/E’ ratio was used as the indicator of LV filling pressure [27] and an independent predictor of cardiac events including heart failure and myocardial infarction [36]. In this study, the increased filling pressure was more prevalent in group 3 and the difference, measured by traditional Doppler analysis of mitral inflow and TDI-derived parameters, was significant.
The E/A ratio was significantly lower in the obese/overweight group compared to the normal-weight group, while E/E’ ratio was significantly higher in the obese/overweight group than the normal-weight group, suggesting an abnormal LV relaxation, with increased dependency on left atrial contraction in the obese/overweight group. The association of these indices with obesity was similarly reported in the previous studies by Çil et al., Russo et al., and Kishi et al. [29-31].
In contrast, Libhaber et al. reported no significant independent relation between E/A and BMI, despite the independent relation between the index of central adiposity (waist circumference) and E/A ratio [33].
LVED, DT, and PWT were found in our study to be significantly correlated with BMI, suggesting an abnormal relaxation and this has been similarly reported by Çil et al., Russo et al., and Kishi et al. [29-31].
While IVRT and RWT were not significantly different among the studied groups, which is consistent with the studies conducted by Çil, et al. and Russo et al. [30, 31].
Moreover, we found no relationship between the LV ejection fraction and obesity, which is consistent with the results of other studies [29-31.
Our results showed that:
- the relationship between diastolic function and BMI parameters is continuous and independent of cardiovascular risk factors associated with obesity, including LV hypertrophy, diabetes, and hypertension; and
- the overweight condition is associated with the impairment of LV diastolic function, close to that observed in obese patients. Actually, there were not any significant differences in most diastolic function parameters between the overweight and obese subjects. Besides, patients with overweight and obesity had a higher risk of pseudonormalized diastolic pattern.
Alpert MA described a pathophysiologic mechanism for the cardiomyopathy of obesity, which begins with increasing the LV volume and cardiac output. These changes may theoretically lead to LVDD and the eventual LV systolic function impairment [19]. Kasper et al. reviewed clinicopathologic findings of all patients who underwent endomyocardial biopsy for dilated cardiomyopathy at a single institution. The percentage of obese patients with idiopathic dilated cardiomyopathy was significantly greater than the lean patients (76.7% vs 35.5%, p=0.001) [37].
Conclusion
Obesity and overweight have a negative independent effect on diastolic function as evaluated by TDI.
Recommendation
WC and BMI should be evaluated more frequently to identify patients who are at risk of LVDD and who are likely to benefit from efficient measures including physical rehabilitation or weight reduction. The TDI technique is almost simple to perform. It has been proven to provide accurate prognostic and diagnostic values in LVDD, and so its regular use is essential in daily practice.
Abbreviation
|
2-dimensional |
2D |
|
body mass index |
BMI |
|
Body surface area |
BSA |
|
peak early diastolic transmitral velocity |
E |
|
peak late diastolic transmitral velocity |
A |
|
peak early diastolic mitral annulus velocity |
E' |
|
Tissue Doppler Imaging |
TDI |
|
Left ventricle |
LV |
|
Ejection fraction |
EF |
|
Waist circumflex |
WC |
|
Weight |
WT |
|
Height |
HT |
|
Left ventricular end systolic dimension |
LVESD |
|
Left ventricular end diastolic dimension |
LVEDD |
|
Left ventricular diastolic dysfunction |
LVDD |
|
Posterior wall thickness |
PWT |
|
Relative wall thickness |
RWT |
|
Isovolumic relaxation time |
IVRT |
|
Deceleration time |
DT |
|
Confidence interval |
CI |
|
Odds ratio |
OR |
|
Cardiovascular disease |
CVD |
|
left atrium |
LA |
|
Left ventricle |
LV |
|
Left ventricular hypertrophy |
LVH |
|
End diastolic volume |
EDV |
|
End diastolic pressure |
EDP |
|
Ischemic heart disease |
IHD |
|
Heart failure |
HF |
|
Left ventricular outflow tract |
LVOT |
|
statistical package for social sciences |
SPSS |
References
- Moyer VA. Screening for and management of obesity in adults: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012 Sep 4;157(5):373-8.
- G.Whitlock, S.Lewington, P.Sherliker, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies Lancet, 373 (2009), pp. 1083-1096.
- Eckel RH, York DA, Rössner S, Hubbard V, Caterson I, St. Jeor ST, Hayman LL, Mullis RM, Blair SN. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: executive summary. Circulation. 2004 Nov 2;110(18):2968-75.
- Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew–Paisley study). European heart journal. 2005 Sep 23;27(1):96-106.
- Yellin EL, Meisner JS. Physiology of diastolic function and transmitral pressure-flow relations. Cardiology clinics. 2000 Aug 1;18(3):411-33.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Journal of Echocardiography. 2016 Jul 15;17(12):1321-60.
- Mitter SS, Shah SJ, Thomas JD. A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. Journal of the American College of Cardiology. 2017 Mar 13;69(11):1451-64.
- Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK. Estimating left ventricular filling pressure by echocardiography. Journal of the American College of Cardiology. 2017 Apr 10;69(15):1937-4
- Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002 Apr 23;105(16):1928-33.
- Schillaci G, Pasqualini L, Verdecchia P, Vaudo G, Marchesi S, Porcellati C, de Simone G, Mannarino E. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. Journal of the American College of Cardiology. 2002 Jun 19;39(12):2005-11.
- Redfield MM, Jacobsen SJ, Burnett Jr JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003 Jan 8;289(2):194-202.
- Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. Journal of the American College of Cardiology. 2001 Mar 15;37(4):1042-8.
- Maeder MT, Buser M, Brenner R, Rickli H. Heart failure with preserved ejection fraction (HFpEF). Therapeutische Umschau. Revue therapeutique. 2018 Sep;75(3):161-9.
- Smiseth OA. Evaluation of left ventricular diastolic function: state of the art after 35 years with Doppler assessment. Journal of echocardiography. 2018 Jun 1;16(2):55-64.
- Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. Journal of the American College of Cardiology. 2008 Feb 19;51(7):679-89.
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. New England Journal of Medicine. 2002 Aug 1;347(5):305-13.
- Loehr LR, Rosamond WD, Poole C, Marie McNeill A, Chang PP, Folsom AR, Chambless LE, Heiss G. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circulation: Heart Failure. 2009 Jan 1;2(1):18-24.
- Palmieri V, de Simone G, Arnett DK, Bella JN, Kitzman DW, Oberman A, Hopkins PN, Province MA, Devereux RB. Relation of various degrees of body mass index in patients with systemic hypertension to left ventricular mass, cardiac output, and peripheral resistance (The Hypertension Genetic Epidemiology Network Study). The American journal of cardiology. 2001 Nov 15;88(10):1163-8.
- Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. The American journal of the medical sciences. 2001 Apr 1;321(4):225-36.
- Norton GR, Majane OH, Libhaber E, Maseko MJ, Makaula S, Libhaber C, Woodiwiss AJ. The relationship between blood pressure and left ventricular mass index depends on an excess adiposity. Journal of hypertension. 2009 Sep 1;27(9):1873-83.
- Varli M, Turhan S, Aras S, Atli T, Erdogan G. Effects of weight loss on ventricular systolic and diastolic functions and left ventricular mass assessed by TDI in obese geriatric women: preliminary report. Aging clinical and experimental research. 2010 Jun 1;22(3):206-11.
- Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of diabetes and hypertension on left ventricular diastolic function in a high‐risk population without evidence of heart disease. European journal of heart failure. 2010 May;12(5):454-61.
- National Institutes of Health. Clinical guidelines for the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. Obes Res. 1998;6(2):51S-209S.
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr 2005;18(12):1440–63.
- Radulescu D, Stoicescu L, Buzdugan E, Donca V. Patterns of left ventricular remodeling among patients with essential and secondary hypertension. Revista medica de Chile. 2013 Dec 1;141(12):1520-7.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. European Journal of Echocardiography. 2009 Mar 1;10(2):165-93.
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and TDI in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000 Oct 10;102(15):1788-94.
- Kossaify A, Nicolas N. Impact of overweight and obesity on left ventricular diastolic function and value of tissue Doppler echocardiography. Clinical Medicine Insights: Cardiology. 2013 Jan;7:CMC-S11156.
- Çil H, Bulur S, Türker Y, Kaya A, Alemdar R, Karabacak A, Aslantaş Y, Ekinözü İ, Albayrak S, Özhan H, MELEN Investigators. Impact of body mass index on left ventricular diastolic dysfunction. Echocardiography. 2012 Jul;29(6):647-51.
- Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology. 2011 Mar 22;57(12):1368-74.
- Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR, Carr JJ, Terry JG, Liu K, Goff DC, Lima JA. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC: Heart Failure. 2014 Oct 1;2(5):500-8.
- Tanalp AC, Bitigen A, Cevik C, Demir D, Ozveren O, Tigen K, Mutlu B, Basaran Y. The role of tissue Doppler study in the assessment of left ventricular dysfunction in obesity. Acta cardiologica. 2008 Oct 1;63(5):541-6.
- Libhaber CD, Norton GR, Majane OH, Libhaber E, Essop MR, Brooksbank R, Maseko M, Woodiwiss AJ. Contribution of central and general adiposity to abnormal left ventricular diastolic function in a community sample with a high prevalence of obesity. The American journal of cardiology. 2009 Dec 1;104(11):1527-
- Canepa M, Strait JB, Abramov D, Milaneschi Y, AlGhatrif M, Moni M, Ramachandran R, Najjar SS, Brunelli C, Abraham TP, Lakatta EG. Contribution of central adiposity to left ventricular diastolic function (from the Baltimore Longitudinal Study of Aging). The American journal of cardiology. 2012 Apr 15;109(8):1171-8.
- Koc F, Tokac M, Kaya C, Kayrak M, Karabağ T, Vatankulu MA, Ayhan S, Demir K. Diastolic functions and myocardial performance index in obese patients with or without metabolic syndrome: a tissue Doppler study. Turk Kardiyoloji Dernegi arsivi: Turk Kardiyoloji Derneginin yayin organidir. 2010 Sep;38(6):400-4.
- Sharp AS, Tapp RJ, Thom SA, Francis DP, Hughes AD, Stanton AV, Zambanini A, O'Brien E, Chaturvedi N, Lyons S, Byrd S. Tissue Doppler E/E′ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. European heart journal. 2009 Nov 26;31(6):747-52.
- Kasper EK, Agema WR, Hutchins GM, Deckers JW, Hare JM, Baughman KL. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. Journal of the American College of Cardiology. 1994 Mar 1;23(3):586-90.
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]