The antiresorptive effect of neostigmine in ovariectomy-induced osteoporosis in rats
Mona O. Abdelhalim1, Hesham M. Mahmoud1, Mira F Y Yacoub2, Abeer S. Mohammad1*
1 Department of Medical Pharmacology, Faculty of Medicine, Cairo University, Egypt. 2 Department of Histology, Faculty of Medicine, Cairo University, Egypt.
Correspondence: Abeer S. Mohammad, Department of Medical Pharmacology, Faculty of Medicine, Cairo University, Egypt. Email: Dr_abeersaid87 @ yahoo.com.
|
ABSTRACT Osteoporosis is a degenerative disease characterized by low bone mass and micro-architectural deterioration of bone tissue, with a consequent increase in susceptibility to fractures. The most common type occurs in postmenopausal women as a result of estrogen deficiency. Autonomic imbalance is supposed to play an important role in the pathophysiology of osteoporosis. This experimental study was designed to investigate the possible curative effects of cholinergic stimulation by Neostigmine on osteoporotic model in adult female albino rats. Forty mature female Wistar rats, weighing (200-250 gm) were used, divided to 5 groups. Group 1 (control group), group 2 (ovariectomized untreated), group 3 (treated with Alendronate 3 mg/kg), group 4 (treated with Neostigmine 12.5 mg/kg), and group 5 (Alendronate+Neostigmine at the same previous doses). Treatment was started after 4 weeks of induction of osteoporosis and lasted for 8 weeks. At the end of treatment, serum Bone specific alkaline phosphatase (BSALP) was measured; histopathological examination of femur, cortical bone thickness measurement & scoring were done to all groups. Neostigmine treatment produced significant decrease in the BSALP level, significant increase in the cortical bone thickness and improvement of histopathological score. Adding Alendronate to Neostigmine (group 5) produced a greater improvement rather than each drug alone. Keywords: Osteoporosis, Ovariectomy, Alendronate, Cholinergic, Rat. |
Introduction
Around 8.9 million fractures a year occur worldwide resulting from osteoporosis [1]. Osteoporosis is characterized by reduced bone mass and altered bone microarchitecture, which results in fragility fractures [2].
The pathophysiological mechanisms of bone loss in osteoporosis include an imbalance between osteoclastic and osteoblastic function, where the osteoclastic bone resorption exceeds the osteoblastic bone formation [3].
Acetylcholine (ACh), besides its important role as neurotransmitter in the nervous system, is signaling molecule in non-neuronal cells [4]. Cholinergic activity has been shown to favor bone mass by increasing osteoblast proliferation and promoting the apoptosis of osteoclasts [5].
Bone cells have been shown to express several components of the cholinergic system (transmitter, enzymes and receptors) [6]. Acetylcholine has been suggested to be produced by osteosblasts due to the presence of the vesicular acetylcholine transporter enzyme in these cells [7].
Cholinergic receptors, both nicotinic and muscarinic, have been identified on the membranes of human primary bone cells, mesenchymal stem cells, osteoblasts and osteoclasts [8]. Also, the mRNA of several nicotinic receptor subtypes (α1, α4, b1 and g) is also present in osteocytes. Among these cholinergic receptors, the nicotinic subtype- α2 receptor and muscarinic-3 receptor appear important in bone physiology. Acetylcholinesterase is expressed in bone cells such as bone marrow-derived monocytes, osteoclasts and osteoblasts [6, 9].
The wide expression of cholinergic components in bone tissue points toward the important role they could play in bone remodeling [4]. A previous study has shown that acetylcholine might regulate the migration of bone marrow-derived multiprogenitor mesenchymal stem cell capable of differentiating into bone cells [10].
It has been shown that bone tissues are innervated by cholinergic fibers of both the parasympathetic and the sympathetic nervous system. Periosteal denervation results in poor bone healing in animal models indicating that periosteal nerves are required for bone formation and fracture healing [11, 12].
Therefore, the aim of this study was to investigate whether boosting acetylcholine activity might have an anabolic effect on bone formation or not. Neostigmine, an anticholinesterase that inhibit acetylcholine breakdown at sites of release, was used in the experimentally induced osteoporotic model to verify this hypothesis.
Materials and Methods
Experimental animals & Ethical statement
Forty female Wistar rats were used in the study. Animals were housed in a room with a 12-h light/dark cycle at a controlled room temperature of 21 ± 2 °C, with food and water access ad libitum. They were acclimatized for one week before the random allocation to the groups. The study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, Egypt. The protocol was approved by the Ethical Committee of Faculty of Medicine, Cairo University, Egypt with approval number CU III S 24 16. All efforts were done to minimize animal suffering.
Osteoporotic model induction
Bilateral ovariectomy (OVX) was done to induce an experimental model of osteoporosis as was originally described by Wronski et al. (1985). Rats were anaesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg) [13]. Two incisions were made beneath the costal margin in the right and left flanks. The ovaries were exteriorized, ligated and excised then the abdomen was closed [14]. Some rats were exposed to sham surgery without removing the ovaries.
Drugs
Neostigmine bromide (Neostig) was supplied in the form of white powder (Sigma-Aldrich Company, USA). Alendronate sodium (ALN) was provided as Fosamax® tablets 10 mg (Merck, Inc.). Tablets were crushed and suspended in sterile water (3 mg/ml) just before oral administration.
Experimental design
Five main groups of rats were included in the study (8 animals each). Sham operated rats were considered as group 1. Ovariectomized rats were left untreated for 4 weeks and then randomly allocated to the following groups. Group 2 or OVX-untreated group: they daily received 1ml of distilled water by gavage for 8 weeks. Group 3: treated with oral alendronate 3 mg/kg daily [15]. Group 4: received neostigmine (12.5mg/kg) every day orally. Group 5: was orally treated daily with alendronate (3 mg/kg) and neostigmine (12.5mg/kg). Treatment of groups 3 – 5 continued for eight weeks.
Measurement of Serum Bone-Specific Alkaline Phosphatase
At the end of the study, blood samples were collected from rat tail veins then centrifuged for serum separation to measure the Bone-Specific Alkaline Phosphatase (BSALP).
Histopathological examination
Animals were sacrificed by using ketamine overdose (300 mg/kg) then both hind limbs were separated and the femurs were decalcified in 10% nitric acid for 6 days. Bones were then fixed in 10% formalin solution for 48 hrs. Thirty micrometer cross-sections of femur were made, dehydrated with alcohol, embedded in xylene and paraffin and cut into 5um thick sections. Each section was stained with Hematoxylin-Eosin (H&E).
The cortical bone thickness was measured in the femur diaphysis by quantitative analysis system, using Leica Qwin 500 software imaging system, in 5 serial sections per animal. Photography was done using DP27 Olympus digital camera and CellSens software system.
Grading of bone affection and/or preservation was done according to the modified scoring protocol of Pritzker et al., (2006) [16]. Histopathological scoring system was done including many parameters which were reduced thickness of cortical bone, presence of fractures, presence of bone defects within thickness, loss of homogenous appearance of matrix, improper arrangement of Haversian systems & periosteal irregularity.
Each parameter was assessed in each group as compared to control values. Control or values similar to control were scored 0 for that parameter, and deviation from the normal was scored as 1, 2 or 3 according to level of affection. Score 0, if pathological change per slide was less than 25%. Score 1, if pathological change per slide was 25%-50%. Score 2, if pathological change per slide was 50%-75%. Score 3, if pathological change per slide was more than 75%.
Scoring for all parameters was then added up for each group, then grading was done according to the mean of the total scores in each group as the following:
Score (0-3) was best assessment= Normal; Score (4- 8) = mild grade of osteoporosis; Score (9-13) = moderate grade of osteoporosis; Score (14-18)= severe grade of osteoporosis.
Statistical analysis
Data were coded and entered using version 23 of the statistical package SPSS. All values were expressed as mean ± SD. Student's t-test was used for comparisons of two groups (normal and ovariectomized). Comparisons between groups were done using analysis of variance (ANOVA) with Tukey's multiple comparisons post hoc test. P-values less than 0.05 were considered as statistically significant [17].
Results
Rats of group 2 (ovariectomized untreated) showed a significant increase in the mean serum BSALP level compared to group 1. Bone histomorphometric analysis revealed marked osteoporotic changes with multiple bone defects (photos 2) and a high histopathological score including significant thinning of the cortex compared to group 1.
On the other hand, rats of group 3 treated with Alendronate showed a significant decrease in the mean serum BSALP level compared to untreated group 2. Moreover, bone histomorphometric analysis revealed marked improvement of bone structure and significant low histopathological score including significant increase in the mean cortical bone thickness compared to that in group 2 (photo 3).
Furthermore, rats of group 4 treated with Neostigmine showed a significant decrease in mean serum BSALP level compared to untreated group 2 and insignificant decrease compared to group 3. Bone histomorphometric analysis revealed marked improvement of bone structure and significant low histopathological score including significant increase in the mean cortical bone thickness compared to untreated group 2 and insignificant increase compared to group 3 (photo 4).
Also, rats of group 5 treated with (Alendronate & Neostigmine) showed a significant decrease in the mean serum BSALP level compared to groups 2, 3 and 4. Bone histomorphometric analysis revealed a significant low histopathological score including significant increase in the mean cortical bone thickness compared to group 2 and insignificant increase compared to groups 3 and 4 (photo 5).
All results were included in tables (1), (2) & (3); figures (1) & (2); photos (1), (2), (3), (4) & (5).
Discussion
Osteoporosis has become a major health hazard disease in recent years, affecting over 200 million people worldwide [18].
Anti-resorptive agents, including bisphosphonate and estrogen, are widely used in treatment of osteoporosis [19]. Unfortunately, evidence indicates that the long term use of estrogen treatment (estrogen replacement therapy) is accompanied by side effects such as increased risk of breast, ovarian and endometrial cancers [20].
Moreover, use of bisphosphonates is associated with esophageal cancer, gastrointestinal problems and osteonecrosis of the jaw [21]. Therefore, there is an urgent demand to search for potential effective pharmacological alternative therapy for osteoporosis disease.
The development and activation of osteoblasts and osteoclasts is controlled by growth factors and cytokines produced by bone cells themselves as well as by surrounding bone marrow cells [22]. More recently, the neuroendocrine system as well as the autonomic nervous system has been implicated in the regulation of bone remodeling [23].
In the present study, deleterious effects on bone with osteoporotic changes were detected four weeks after surgery in the groups underwent ovariectomy. This was evidenced by histomorphometric analysis of rat femurs which revealed marked osteoporotic changes in the form of appearance of widened cavities of haversian canals, pale matrix and bone defects. Then treatment was started with alendronate, neostigmine and their combinations for eight weeks. At the end of experiments, serum BSALP level was measured as well as histomorphological analysis including computation of cortical bone thickness and histological scoring were done to all groups.
Ovariectomy induced osteoporosis in untreated group 2 (OVX untreated) resulted in a significant increase in BSALP level as well as marked osteoporotic changes with a significant increase in the histopathological score including reduction of the mean cortical thickness, as one of the parameters assessed, compared to the control group 1. This could be explained as ovariectomy model in rats’ mimics estrogen deficiency in humans; this estrogen deficiency increases osteoclast formation and therefore supports bone resorption. Shortage of estrogen also induces apoptosis of osteoblasts [24]. This leads to an imbalance between osteoblastic bone formation and osteoclastic bone resorption, favoring bone loss and resulting in the osteoporotic changes and bone fractures [25].
On the other hand; after menopause, bone resorption markers increase due to the activation of bone resorption by estrogen deficiency, and bone formation markers increase as a compensatory mechanism to fill the higher number of resorption cavities, and as a result, increase in their serum levels. BSALP, a bone formation marker, is found in the membrane of osteoblasts and released into the circulation during bone formation [26].
A large cross sectional study was done by Pedrazzoni et al. (1996) to examine the serum levels of BSALP and its relation to osteoporosis [27]. It was found that serum level of BSALP was significantly increased in postmenopausal women and in women underwent ovariectomy.
Moreover, Alam et al. (2006) detected significant elevation of BSALP in ovariectomized rats as compared to sham group [28]. Also, Shin et al. (2012) and Sun et al. (2013) worked on ovariectomized rats with detection of higher levels of BSALP in OVX group indicating increased bone turnover due to estrogen deficiency [29, 30].
Furthermore, Mukaiyama et al. (2015) and Abuohashish et al. (2015); all confirmed that in OVX rats, the levels of bone turnover biomarkers (BALP) were significantly elevated [31, 32]. Moreover, cortical and trabecular morphometric parameters and histopathology of femoral bones were severely altered by ovariectomy.
Also, Palumbo et al. (2009) studied histological observations of bone resections of vertebrae and femurs with detection of obvious bone mass reduction in ovariectomized rats [33]. Similarly,Yoon et al. (2012) observed that cortical bone mineral density was significantly lower in the ovariectomized group [34].
In the present work, Alendronate was administered to rats of group 3 in a dose of 3 mg/kg/day for 8 weeks and resulted in a significant reduction in the mean serum BSALP level, significant increase in the mean cortical bone thickness and a significant improvement of histopathological score when compared to untreated group 2.
Alendronate is a drug that belongs to bisphosphonate family, approved by the FDA for the prevention and treatment of postmenopausal osteoporosis. Moreover, it is approved to reduce the incidence of vertebral as well as hip fracturers. It acts by binding to hydroxyapatite crystals in bone and inhibiting osteoclast mediated bone resorption. Also, it decreases matrix breakdown in bone.
Various animal studies support this significant improvement in the laboratory profile upon treatment with alendronate. Results of this study were matched with Pedrazzoni et al. (1996) as they observed a significant reduction in the serum bone specific alkaline phosphatase (BSALP) level after alendronate treatment [27].
Also, Mukaiyama et al. (2015) have found that the alkaline phosphatase (ALP) and bone specific alkaline phosphatase (BSALP) serum levels of people in their 80s were significantly higher than those of people in their 60s [31]; And with bisphosphonate therapy, the serum BSALP decreased to normal range levels.
Furthermore, Chavassieux et al. (1997) confirmed that treatment with alendronate has been associated with histomorphometric improvement of bone mass and structure in the form of a significant increase in bone thickness accompanied by a reduction in erosion depth as well as a marked decrease in trabecular bone turnover [35].
On the other hand, in the present work, therapeutic dose of neostigmine (12.5/mg/kg/day) was administered to rats of group 4 for 8 weeks. Neostigmine treatment resulted in a significant reduction of mean serum BSALP level, significant increase of cortical bone thickness and significant improvement of histopathological score when compared to the untreated group 2.
Shi et al. (2010) explained this improvement by demonstrating that activation of the parasympathetic nervous system decreases bone resorption and increases bone formation [5]. This results in an improvement of bone mass through release of the parasympathetic neurotransmitter acetylcholine (ACh) and binding to muscarinic acetylcholine receptors (mAChR) of the subtype M3 (M3 mAChR).
In addition to having a neuronal origin, ACh can be secreted by a multiplicity of non-neuronal cells like endothelial cells, immune cells, and mesenchymal cells [4]. It binds in an auto-paracrine manner (like a hormone) on acetylcholine receptors and enhances proliferation, differentiation, and maintenance of cell contacts of many cell types, including osteoblasts [8, 36].
M3mAChR is expressed by osteoblasts. Besides M3 mAChR, 4 other subtypes of mAChR were identified (M1-M5 mAChR) [37] and expressed in bone tissue, especially in osteoblasts [7, 8, 36] . Upon activation, mAChR couple to G-proteins to regulate ion channel permeability and second messenger signaling pathways. Acetylcholine binding to M3 mAChR results in an elevation of cytosolic calcium concentrations and proliferation of osteoblast cells [7, 8].
Shi et al. (2010) & Kliemann et al. (2012) all proved that mice with a homozygous gene deficient in M3 mAChR showed a significant reduction of bone bending stiffness, relative bone volume, and alterations of the trabecular microstructure [5, 38]. Therefore, they suggested that M3 mAChR is involved in the regulation of bone mass & consequently, loss of M3 mAChR can lead to a decrease in bone mass and deterioration of bone microstructure.
Moreover, (Shi and his colleagues (2010) demonstrated that a regulating role of M1, M2, and M4 mAChR in bone remodeling is somewhat unlikely because mice that are gene deficient for these receptors did not show any bone phenotype change [5]. Bone of M5 mAChR knockout mice has not been investigated until now. M3 mAChR and M5 mAChR belong together to Gaq/11 coupled proteins that enhance the intracellular calcium content and therefore are an interesting target for investigations.
To our best knowledge, no animal studies were conducted to demonstrate the effect of neostigmine (choline estrase inhibitor) on osteoporosis. However, in (2015) Lips and his colleagues confirmed that bone loss has already been determined in male mice with gene deficiency of muscarinic acetylcholine receptor M3 (M3R-KO) [39]. They found reduced biomechanical strength of M3R-KO correlated with decreased cortical thickness and decreased bone mineral density (BMD). Thus, they, appearing in accordance with the results of the present study, concluded that their results on bone properties of 16-week old female M3R-KO are related to postmenopausal osteoporotic phenotype & Stimulation and up-regulation of muscarinic acetylcholine receptor subtype M3 expression in osteoblasts might be a possible new option for prevention and therapy of osteoporotic fractures.
Moreover, Lips et al. (2014) support the importance of the parasympathetic innervation of bone and its potential role in improvement of healing in an ovariectomy induced osteoporosis animal model [40]. Thus, this study is in accordance with their work, both share the rule of the importance of cholenergic nerve supply in bone formation and thereby bone healing.
Furthermore, Eimar et al. (2013) summarized several observations demonstrating that the cholinergic signaling is a positive regulator of bone mass [41]. In vitro studies have suggested that acetylcholine might regulate proliferation and differentiation of bone cells. They added that in vivo studies have shown that altering cholinergic activity regulates bone mass accrual. These observations appear in agreement of several clinical studies that have shown that diseases caused by disruption of cholinergic activity seem to be associated with bone disorders. So they concluded that their work presented a substantial evidence indicating the importance of cholinergic activity on bone, however there is still much to learn about the complicated relationships between the cholinergic system and bone. Their work appears in accordance with this study which showed a significant improvement of biochemical and histomorphological results upon treatment of rats with neostigmine.
Also, Tosun et al. (2011) demonstrated the presence of autonomic dysfunction in patients with osteoporosis through a prospective controlled trial [42]. They concluded that autonomic dysfunction characterized with increased sympathetic and decreased parasympathetic activity may be present in osteoporosis, and cardiac functions in patients with osteoporosis may also be affected by accompanying autonomic dysfunction.
Furthermore, the combination treatment of alendronate (3mg/kg/day) and neostigmine (12.5mg/kg/day) in group 5 for 8 weeks was analyzed in the present study. This combination treatment produced a significant reduction in the mean serum BSALP level when compared to the corresponding values in untreated group 2, alendronate treated group 3 and neostigmine treated group 4.
Moreover, this combination resulted in a significant lower histopathological score that include a significant increase in the mean cortical bone thickness when compared to untreated group2. However, there was an insignificant increase when compared to alendronate treated group 3 and neostigmine treated group 4.
The significant reduction in the mean serum BSALP level compared to groups 3 & 4 could be due to the hypothesis of the complementary modes of action of both drugs that resulted in a greater antiosteoporotic effect more than anyone alone. However, the BSALP level is not a strong indicator for bone thickness as it lacks specificity and by itself, is not sufficient.
Conclusion
To our knowledge, this is the first study conducted to demonstrate the effect of neostigmine (choline estrase inhibitor) added to bisphosphonate therapy on osteoporosis and no experimental studies were done on this combination treatment for osteoporosis.
From the present work, it could be concluded that Neostigmine has antiosteoporotic effect as was evident by laboratory decrease in the serum BSALP level, histopathological improvement in the bone structure, increase in the cortical bone thickness and low histopathological score.
Also, the present work demonstrated that the combination of Alendronate and Neostigmine has a greater antiosteoporotic effect than each drug alone.
So, this work suggests that greater attention should be paid to Neostigmine in addition to bisphosphonates in the field of treatment of osteoporosis in postmenopausal women.
Recommendation:
It could be said that this interesting point needs a further investigation and analysis in future research, including studies on Neostigmine, alone and combined, in variable doses, both experimentally and clinically in order to confirm the present results and reach a definite, well understood and effective treatment for osteoporosis.
References
- Cruz AS, Lins HC, Medeiros RVA, Filho JMF, da Silva SG (2018). Artificial intelligence on the identification of risk groups for osteoporosis, a general review. BioMedical Engineering OnLine 17(1): 12.
- Grases F, Sanchis P, Prieto RM, Perelló J, López-González ÁA (2010). Effect of Tetracalcium Dimagnesium Phytate on Bone Characteristics in Ovariectomized Rats. Journal of Medicinal Food 13(6): 1301-1306.
- Potu BK, Rao MS, Nampurath GK, Chamallamudi MR, Prasad K, Nayak SR, et al. (2009). Evidence-based assessment of antiosteoporotic activity of petroleum-ether extract of Cissus quadrangularis Linn. on ovariectomy-induced osteoporosis. Upsala Journal of Medical Sciences 114(3): 140-148.
- Wessler I, Kirkpatrick C (2008a). Acetylcholine beyond neurons: the non‐neuronal cholinergic system in humans. British journal of pharmacology 154(8): 1558-1571.
- Shi Y, Oury F, Yadav VK, Wess J, Liu XS, Guo XE, et al. (2010a). Signaling through the M 3 muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell metabolism 11(3): 231-238.
- Bajayo A, Bar A, Denes A, Bachar M, Kram V, Attar-Namdar M, et al. (2012). Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proceedings of the National Academy of Sciences of the United States of America 109(38): 15455-15460.
- Sato T, Abe T, Chida D, Nakamoto N, Hori N, Kokabu S, et al. (2010a). Functional role of acetylcholine and the expression of cholinergic receptors and components in osteoblasts. FEBS letters 584(4): 817-824.
- Liu P-S, Chen Y-Y, Feng C-K, Lin Y-H, Yu T-C (2011). Muscarinic acetylcholine receptors present in human osteoblast and bone tissue. European journal of pharmacology 650(1): 34-40.
- Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, et al. (2009). Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 45(4): 682-692.
- Tang J-M, Yuan J, Li Q, Wang J-N, Kong X, Zheng F, et al. (2012). Acetylcholine induces mesenchymal stem cell migration via Ca2+ /PKC/ERK1/2 signal pathway. Journal of cellular biochemistry 113(8): 2704-2713.
- Asmus SE, Parsons S, Landis SC (2000). Developmental Changes in the Transmitter Properties of Sympathetic Neurons That Innervate the Periosteum. The Journal of Neuroscience 20(4): 1495-1504.
- Song D, Jiang X, Zhu S, Li W, Khadka A, Hu J (2012). Denervation impairs bone regeneration during distraction osteogenesis in rabbit tibia lengthening. Acta orthopaedica 83(4): 406-410.
- Wronski TJ, Lowry PL, Walsh CC, Ignaszewski LA (1985). Skeletal alterations in ovariectomized rats. Calcified Tissue International 37(3): 324-328.
- Waynforth HB, Flecknell PA (1980). Experimental and surgical technique in the rat. edn, vol. 127. Academic press London.
- Cegieła U, Pytlik M, Folwarczna J, Miozga R, Piskorz S, Nowak D (2014). Exercise prevented the lansoprazole-induced reduction of anti-osteoporotic efficacy of alendronate in androgen deficiency rats. Acta Pol Pharm 71(3): 485-495.
- Pritzker, K.P.H., Gay, S., Jimenez, S.A., Ostergaard, K., Pelletier, J.P., … Van den Berg, W.B. (2006): Osteoarthritis cartilage histopathology: grading and staging. OsteoArthritis and Cartilage; 14: 13-29.
- Chan, H. (2003): Upper Bounds and Importance Sampling of p-Values for DNA and Protein Sequence Alignments," Bernoulli, 9, 183-199.
- Rachner TD, Khosla S, Hofbauer LC (2011). Osteoporosis: now and the future. Lancet (London, England) 377(9773): 1276-1287.
- Chen JS, Sambrook PN (2011). Antiresorptive therapies for osteoporosis: a clinical overview. Nature Reviews Endocrinology 8: 81.
- Morrow R, Deyhim F, Patil BS, Stoecker BJ (2009). Feeding orange pulp improved bone quality in a rat model of male osteoporosis. Journal of medicinal food 12(2): 298-303.
- Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. (2011). Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: Executive summary of recommendations from the American Dental Association Council on Scientific Affairs. The Journal of the American Dental Association 142(11): 1243-1251.
- Seeman E, Delmas PD (2006). Bone Quality — The Material and Structural Basis of Bone Strength and Fragility. New England Journal of Medicine 354(21): 2250-2261.
- Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V, et al. (2008). Low dose beta-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. Journal of Cellular Physiology 217(3): 819-827.
- Manolagas S, Kousteni S, Jilka R (2002). Sex steroids and bone. Recent progress in hormone research 57: 385-409.
- McNamara L (2009). Perspective on post-menopausal osteoporosis: Establishing an interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. edn, vol. 7.
- Singer FR, Eyre DR (2008). Using biochemical markers of bone turnover in clinical practice. Cleveland Clinic journal of medicine 75(10): 739-750.
- Pedrazzoni M, Alfano F, Girasole G, Giuliani N, Fantuzzi M, Gatti C, et al. (1996). Clinical observations with a new specific assay for bone alkaline phosphatase: A cross-sectional study in osteoporotic and pagetic subjects and a longitudinal evaluation of the response to ovariectomy, estrogens, and bisphosphonates. Calcified tissue international 59(5): 334-338.
- Alam MR, Kim SM, Lee JI, Chon SK, Choi SJ, Choi IH, et al. (2006). Effects of Safflower seed oil in osteoporosis induced-ovariectomized rats. The American journal of Chinese medicine 34(04): 601-612.
- Shin Y-H, Cho D-C, Yu S-H, Kim K-T, Cho H-J, Sung J-K (2012). Histomorphometric analysis of the spine and femur in ovariectomized rats using micro-computed tomographic scan. Journal of Korean Neurosurgical Society 52(1): 1-6.
- Sun G, Guo T, Chen Y, Xu B, Guo J, Zhao J (2013). Significant pathways detection in osteoporosis based on the bibliometric network. Eur Rev Med Pharmacol Sci 17(1): 1-7.
- Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H (2015). Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging clinical and experimental research 27(4): 413-418.
- Abuohashish HM, Ahmed MM, Al-Rejaie SS, Eltahir KE (2015). The antidepressant bupropion exerts alleviating properties in an ovariectomized osteoporotic rat model. Acta Pharmacologica Sinica 36(2): 209.
- Palumbo C, Ferretti M, Bertoni L, Cavani F, Resca E, Casolari B, et al. (2009). Influence of ferutinin on bone metabolism in ovariectomized rats. I: role in preventing osteoporosis. Journal of Bone and Mineral Metabolism 27(5): 538-545.
- Yoon K-H, Cho D-C, Yu S-H, Kim K-T, Jeon Y, Sung J-K (2012). The change of bone metabolism in ovariectomized rats: analyses of microCT scan and biochemical markers of bone turnover. Journal of Korean Neurosurgical Society 51(6): 323.
- Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ (1997). Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. The Journal of clinical investigation 100(6): 1475-1480.
- En-Nosse M, Hartmann S, Trinkaus K, Alt V, Stigler B, Heiss C, et al. (2009). Expression of non-neuronal cholinergic system in osteoblast-like cells and its involvement in osteogenesis. Cell and tissue research 338(2): 203-215.
- Eglen R (2006). Muscarinic receptor subtypes in neuronal and non‐neuronal cholinergic function. Autonomic and Autacoid Pharmacology 26(3): 219-233.
- Kliemann K, Kneffel M, Bergen I, Kampschulte M, Langheinrich AC, Dürselen L, et al. (2012). Quantitative analyses of bone composition in acetylcholine receptor M3R and alpha7 knockout mice. Life sciences 91(21-22): 997-1002.
- Lips KS, Kneffel M, Willscheid F, Mathies FM, Kampschulte M, Hartmann S, et al. (2015). Altered ultrastructure, density and cathepsin K expression in bone of female muscarinic acetylcholine receptor M3 knockout mice. International immunopharmacology 29(1): 201-207.
- Lips K, Kauschke V, Hartmann S, Thormann U, Ray S, Schumacher M, et al. (2014). Cholinergic nerve fibers in bone defects of a rat osteoporosis model and their regulation by implantation of bone substitution materials. J Musculoskelet Neuronal Interact 14(2): 173-188.
- Eimar H, Tamimi I, Murshed M, Tamimi F (2013). Cholinergic regulation of bone. Journal of musculoskeletal & neuronal interactions.
- Tosun A, Dogru MT, Aydn G, Keles I, Arslan A, Güneri M, et al. (2011). Does autonomic dysfunction exist in postmenopausal osteoporosis? American journal of physical medicine & rehabilitation 90(12): 1012-1019.
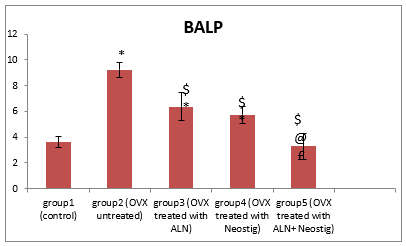
Figure 1: Changes in the mean bone specific alkaline phosphatase BSALP after oral treatment with Alendronate 'ALN' (3 mg/kg/day), Neostigmine (12.5 mg/kg/day) & their combinations in ovariectomy-induced osteoporosis (n= 8)
* Significant change compared to group 1
$ Significant reduction compared to group 2
@ Significant reduction compared to group 3
£ Significant reduction compared to group 4
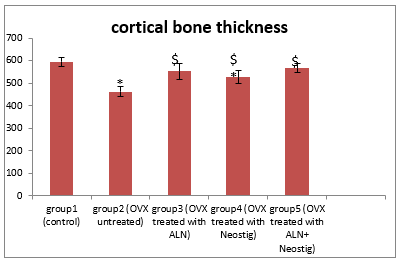
Figure2: Changes in the mean cortical bone thickness after oral treatment with Alendronate 'ALN' (3 mg/kg/day), Neostigmine (12.5 mg/kg/day) & their combinations in ovariectomy-induced osteoporosis (n= 8)
* Significant change compared to group 1
$ Significant increase compared to group 2
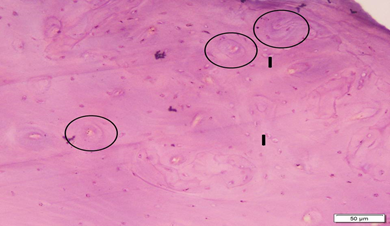
Photo 1A: Control group 1 shows normal cortical bone with homogenous complete areas of bone tissue. Circles mark osteons (Haversian systems). Note the compact arrangement of osteons, and the interstitial lamellae (I). (H&E×200).
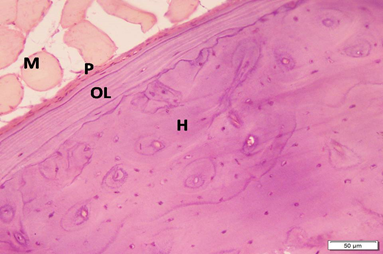
Photo 1B: Control group 1 shows periosteum (P), outer circumferential bone lamellae (OL), and Haversian systems (H). Note the skeletal muscle (M) attached to outer surface of bone (H&E×200).
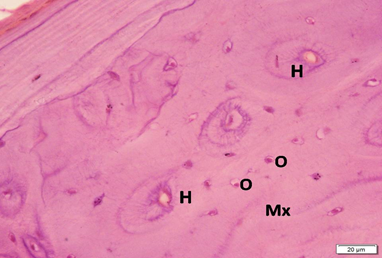
Photo 1C: Control group 1 shows blood vessels inside Haversian canals (H) and osteocytes (O) in concentric layers filling all field of the acidophilic bone matrix (Mx) (H&E×400).
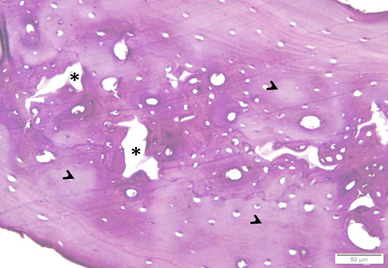
Photo 2A: Ovariectomized untreated group 2 shows many widened cavities (*) inside a wide patch of bone. Matrix is pale in many areas (arrowheads) (H&E×200).
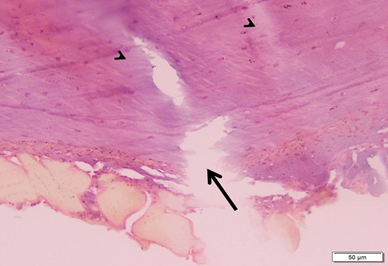
Photo 2B: Ovariectomized untreated group 2 shows a partial break (arrow) starting from outer surface of bone, together with non homogenous staining of matrix (arrowheads) (H&E×200).
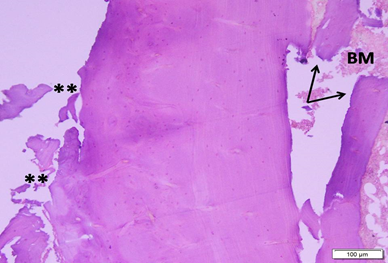
Photo 2C: Ovariectomized untreated group 2 shows bone deformity in the form of loss of smooth outer contour (**) and breaks (arrow) on the inner surface near the bone marrow (BM).
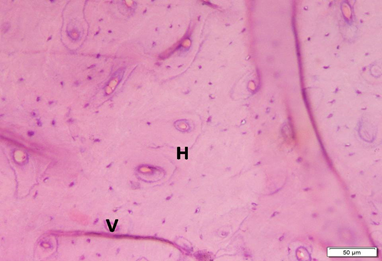
Photo 3A: Effect of Alendronate treatment in group 3 shows dense compact bone, with many osteons (Haversian systems) and interstitial lamellae. Transverse Volkmanns canals (V) are also evident (H&E×200).
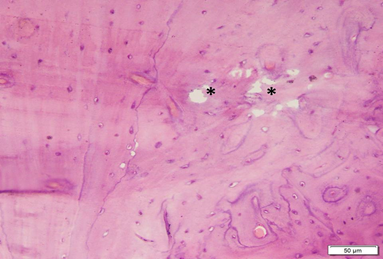
Photo 3B: Effect of Alendronate treatment in group 3 shows well formed bone, except for small areas of erosion (*) within the bone (H&E×200).
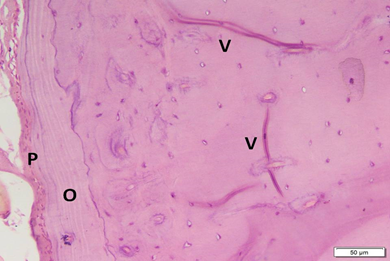
Photo 3C: Effect of Alendronate treatment in group 3 shows increased thickness of periosteum (P) (i.e. increased activity), with mild irregularity of the outer surface, and also increased thickness of outer circumferential bone lamellae (O). Volkmanns canals are evident (H&E ×200).
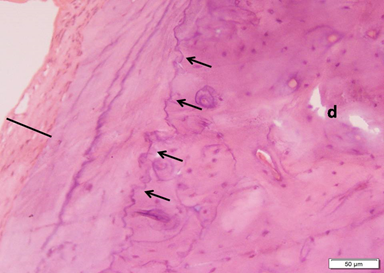
Photo 4A: Effect of Neostigmine treatment in group 4 shows markedly thickened periosteum (black line), more regular demaraction lines (small arrows) and few small bone defects (d) within the thickness (H&E ×200).
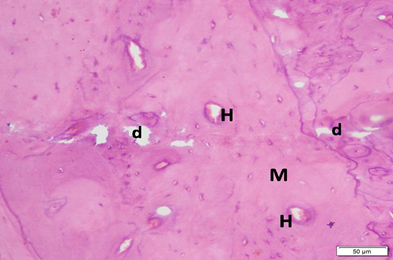
Photo 4B: Effect of Neostigmine treatment in group 4 shows thick bone, with overall regular arrangement and homogenous matrix (M). Haversian canals (H) can be seen, but there are several distributed small bone defects (d) (H&E ×200).
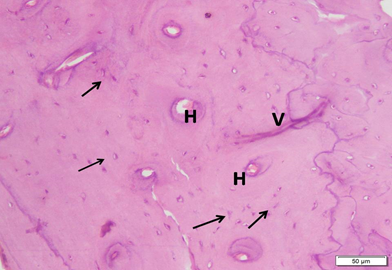
Photo 4C: Effect of Neostigmine treatment in group 4 shows thick regular arrangement of the compact bone, with regular Haversian canals (H), Volkmanns canal (V) and osteocytes in lacunae (arrows) filling the area. No bone defects can be seen (H&E ×200).
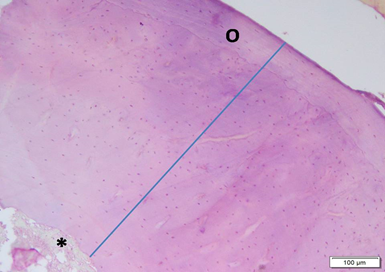
Photo 5A: Effect of combined Alendronate & Neostigmine treatment in group 5 shows large areas of well formed thick bone (blue line) with no defects. Outer circumferential lamellae (O) are evident and regular. Note bone marrow cavity (*) at lower left corner (H&E ×100).
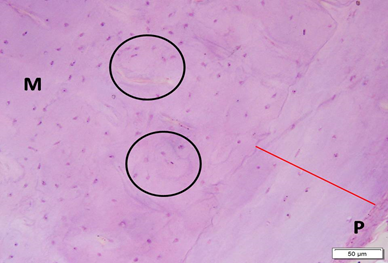
Photo 5B: Effect of combined Alendronate & Neostigmine treatment in group 5 shows outer circumferential lamellae (red line) underneath periosteum (P) and Haversian systems (circled areas), all in compact matrix (M) with no empty spaces or gaps (H&E ×200).
|
Table 1: Effect of oral treatment with Alendronate 'ALN' (3 mg/kg/day), Neostigmine (12.5 mg/kg/day) & their combinations on serum Bone Alkaline Phosphatase level |
||||||
|
|
Mean (IU/L) ±SD |
Percent change compared to Group 1 |
Percent change compared to Group 2 |
Percent change compared to Group 3 |
Percent change compared to Group 4 |
Percent change compared to Group 5 |
|
Group 1 (control Group) |
3.62±0.43 |
----- |
154.9%* |
75.4%* |
58.2%* |
9.3%# |
|
Group 2 (OVX untreated Group) |
9.23±0.59 |
154.9%* |
----- |
45.3%* |
65.1%* |
181.4%* |
|
Group 3 (ALN treated Group) |
6.35±1.09 |
75.4%* |
-31.2%* |
----- |
10.8%# |
93.5%* |
|
Group 4 (Neostigmine treated Group |
5.73±0.68 |
58.2%* |
-37.9%* |
-9.7%# |
----- |
74.6%* |
|
Group 5 (ALN + Neostigmine treated Group) |
3.28±1.02 |
-9.3%# |
-64.4%* |
-48.3%* |
-42.7%* |
----- |
Data are presented as mean ±SD
*: Significant change (p< 0.05)
#: Insignificant change (p> 0.05)
|
Table 2: Effect of oral treatment with Alendronate 'ALN' (3 mg/kg/day), Neostigmine (12.5 mg/kg/day) & their combinations on cortical bone thickness |
||||||
|
|
Mean (µm)± SD |
Percent change compared to Group 1 |
Percent change compared to Group 2 |
Percent change compared to Group 3 |
Percent change compared to Group 4 |
Percent change compared to Group 5 |
|
Group 1 (control Group) |
593.96±18.56 |
-----
|
28.4%* |
7.5%# |
12.8%* |
4.7%# |
|
Group 2 (Sham Group) |
462.40±21.28 |
-22.1%* |
----- |
-16.3%* |
-12.1%* |
-18.4%* |
|
Group 3 (ALN treated Group) |
552.47±34.35 |
-6.9%# |
19.4%* |
----- |
4.9%# |
-2.5%# |
|
Group 4 (Neostigmine treated Group |
526.39±28.94 |
-11.3%* |
13.8%* |
-4.7%# |
----- |
-7.1%# |
|
Group 5 (ALN + Neostigmine treated Group) |
566.79±19.14 |
-4.5%# |
22.5%* |
2.5%# |
7.6%#
|
----- |
Data are presented as mean ±SD
*: Significant change (p< 0.05)
#: Insignificant change (p> 0.05)
|
Table 3: Histopathological scoring in the studied groups (n=8) (Mean & SD). |
||
|
|
Mean |
Standard Deviation |
|
group1 (control) |
.00 |
± .00 |
|
group2 (OVX untreated) |
17.86 * |
± .90 |
|
group3 (OVX treated with ALN) |
4.00 *$ |
± .82 |
|
Group4 (OVX treated with Neostig) |
6.86 *$@ |
± .90 |
|
Group5 (OVX treated with ALN+ Neostig) |
3.00 *$@ £ |
± .00 |
* Significant change compared to group 1
$ Significant change compared to group 2
@ Significant change compared to group 3
£ Significant change compared to group 4
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]