Regulation of drug transmission within the matrices of different polymers and the models of drug delivery
Hassan Ali Alhmoud
Department of pharmaceutical sciences, college of pharmacy, Yarmouk University, P.O. Box: 566, Irbid, Jordan.
ABSTRACT
The polymers are applied widely in the preparation of controlled release dosage forms. The model of drug release provides important information about the mechanism of their effect on drug release and clarifying the method of drug transmission within the tablets in comparison with its macroscopic appearance. The article will discuss the structure, charge, physicochemical properties, and concentration of polymer on the rate and the mechanism of drug release. The properties of the drug, the dissolution medium, and the other ingredients and their effect on drug release will be discussed. Moreover, the article will provide information about their uses in pharmacy and medicine. The article's goal is to focus on the effects of mixing different polymers with drugs and other ingredients on the preparation of controlled release dosage forms and to elucidate the drug release mechanism.
Keywords: polymers, controlled release, drug release mechanism, macroscopic appearance.
Introduction
Polymers are widely used in our daily life [1] and classified into many classes according to their composition, as well as their physical basis [2]. The monomers are the basic property of the polymer. Other properties such as the microstructure, the polymer size, and length of the chain play an essential role in determining the physical properties and predict the polymer behavior [3, 4]. Altering the microstructure of polyurethane urea material (PUU) identified the micromechanisms governing mechanical behavior and improved the effects of the varying microstructure of the material [5].
The microspheres of Polylactic co- glycolic acid (PLGA) are controlled release drug delivery, releasing drug molecules over long periods of time and maintain drug concentrations in its effective range, and improve patient compliance as compared to conventional regimens [6]. PLGA has been used as controlled-release dosage forms [7-11] because they are biodegradable and bioabsorbable which facilitate degradation and resorption of the polymer in aqueous dissolution medium and the living tissues [12, 13].
Hydroxypropylmethylcellulose (HPMC) is used in the formulation of hydrophilic matrices to obtain controlled release drugs. It is a water-soluble, hydrophilic, nonionic cellulose and stable over the pH range of 3 to 11 and is enzyme resistant [14, 15]
The different physiological parameters of the gastrointestinal fluid affect the release of quetiapine fumarate. These results indicate the importance of the gastrointestinal tract in designing controlled release formulations. [16, 17]
The privilege of controlled release products in the pharmaceutical field are well known [18-20] and includes the ability to maintain the desired blood level of a drug over an almost longer time [21]. Currently, some imaging techniques are used in the field of pharmaceutics to determine the drug release. These imaging techniques include near Infra-red (NIR), Magnetic Resonance (MRI), and Nuclear Magnetic Resonance (NMR) [22]. Others have also been utilized in a variety of pharmaceutical applications [23]. Another work reports methods to determine matrices swelling in real-time Fig.1. This work can provide the behavior to choose the appropriate method of drug release.
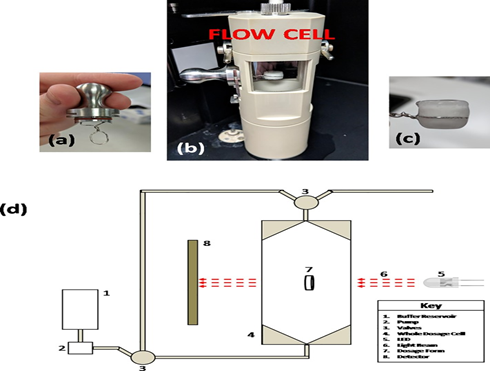
Fig.1. (a) Image of wire tablet holder designed for holding the tablet for swelling studies, (b) tablet holder inserted into the flow cell for image analysis, (c) image of swollen tablet after data acquisition in the flow cell of SDI2 instrument, (d) schematic representation of the surface dissolution imaging instrument (SDI2) with a key inserted in the figure.
Drug release from polymers occurs by:
1 - diffusion-controlled systems: from the hydrophilic and enhance the diffusion of drug from the polymers [24-30](Fig.2).
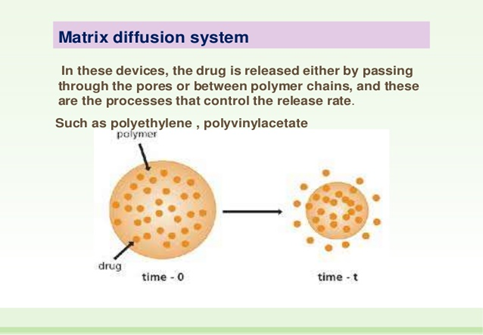
Figure 2: Illustrate the drug release from a matrix of a hydrophilic polymer by the mechanism of diffusion
2 - Erosion-controlled systems: It is from the hydrophobic polymers [8, 31-34] (Fig.3).
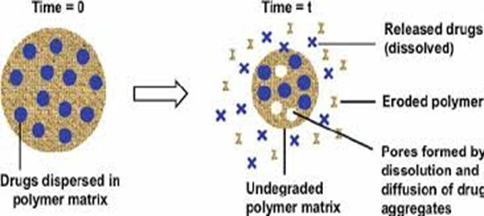
Figure 3: Illustrate the drug release from a matrix of a hydrophobic polymer by the mechanism of erosion
3 – A mixture of different mechanisms aforementioned above has also been used to obtain a controlled release drug system [29, 30, 35-37] (Fig. 4).
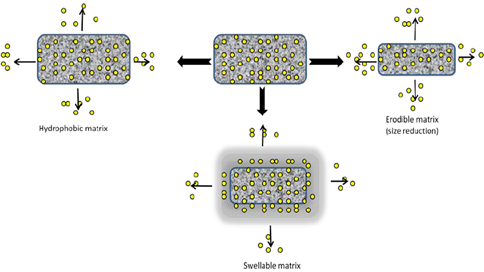
Figure 4: Illustrate the drug release from a matrix with a mixture hydrophilic and hydrophobic polymers by the mechanism of diffusion and erosion.
The incorporation of the حolymer within the matrices may emerge from the therapeutic benefit of the polymer in addition to that of the drug [38] or biodegraded [39]. Polymers can change drug release and half-life characteristics [36, 40, 41]. The use of polymers resulted in producing tablets that facilitate the delivery of new cytoplasmic drug therapeutics [42-44].
The polymer devices are classified as diffusion-controlled, swelling [45], and biodegradable systems [46].
Drug transport
When the matrices were subjected to the dissolution mediumو more of drug released from the matrices in the early stage from the external boundaries of the controlled-release tablets. This phenomenon has been observed in almost all the experiments done during the previous studies [30, 32-37, 40]. That was due to the high and also extra amount of drug on the external boundaries of the tablets. [29, 37].The initial break of the boundaries can be adjusted by the fabrication technique [47-49] for example the drug sometimes is released from the matrices by diffusion or diffusion through the aqueous pores formed during dissolution at the same time with the polymer dissolution [29, 37, 50].
For highly water-soluble drugs, such as proteins and peptides, diffusion is the mode of transport [51]. For controlled-release with hydrophobic polymer matrices used drug release occurred by attrition or erosion of the matrices without mass transport, which is called eroding polymers. It must be taken into consideration that some drug compounds have low water solubility [52, 53]. So, it is necessary to treat small and large drug molecules and to account for transport before and after the pore formed and the size increased. After the pore size increased the drug release from the matrices increases rapidly.
In general, the dissolution of polymer involves two transport processes, i.e. solvent diffusion and chain disentanglement [29, 36, 53]. Polysaccharides such as sodium carboxymethylcellulose (NaCMC), are stable under physiological pH and temperature, but hydrolyze at extreme pH and temperatures.
Mathematical models to predict the drug release
Prediction of drug release profiles by mechanistic models is useful for understanding mechanisms and designing drug release particles [54]. In general, diffusion, erosion, and degradation are the mechanisms for solute release from the matrices [55-57]. The basic and most common mathematical models used are:
1 - Zero-order equation: C = k0 t,
Where C = the cumulative of drug amount release versus time t and k0 is the zero-order rate constant.
2 – First-order release: Log c = log c0 - K1t/2.303.
Drug remaining plotted versus time t; and K1 is constant.
3 - Higuchi equation Q = KH t½
Where Q = cumulative percentage of drug release plotted versus time t and KH is the Higuchi dissolution constant.
4 - Korosmeyer- Peppas equation Mt/M∞ = Mᵏᵖtⁿ [58].
Where Mt/M∞ is the release rate constant. The n is the different release mechanisms. The value for n depends on the type of transport, geometry, and the rate of chain relaxation [59, 60].
The equation describes a drug release from a polymeric system and the values of 60% drug release were fitted in Korosmeyer-Peppas model [60, 61].
5 - Hixson- Crowell's Model Qₒ⅓ - Q⅓ = Kᵸᶜt
Where Q is the drug released in time t, Qₒ is the drug in the tablet, and Kᵸᶜ is the constant rate for the Hixson and Crowell rate equation.
The aforementioned equations are applied for a range of devices [62, 63].
The pharmacy and medicine polymers are used as a suture material for a long time [64], also for hemodialysis or as drug delivery systems for responsive drug release [65]. Biodegradable polymers remain in the body only to accomplish their function, then they disappear without the need for a second surgical intervention [66-68]. Degradable polymers have been further used for drug delivery along with the degradation from microcarriers or macroscopic applications [69, 70]. The polymers as micro- and nanospheres are used for targeted drug delivery (solid colloids, dendrimers, micelles, nanogels, capsules or core–shell particles) [71-73].
Conclusion
The physicochemical properties of polymers, their concentrations, and structure importantly indicate the rate of drug release from the matrices. The imaging techniques also showed different mechanisms of drug release from the matrices which will be an assistant factor in the prediction of drug release. The drug, other excipients, and the dissolution medium play a helpful role in regulating the drug release from the matrices. Responsive degradation of polymers upon defined triggers also allows controlled drug release applications.
Acknowledgment
The author would acknowledge Yarmouk University for its support for the work.
References
- McCrum, N. G.; Buckley, C. P.; Bucknall, C. B. Principles of polymer engineering, Oxford; New York: Oxford University Press. 1997; P.
- Baeurle, S. A. Multiscale modeling of polymer materials using fieldtheoretic methodologies: a survey about recent developments. Journal of Mathematical Chemistry, 2009; 46 (2): 363–426.
- Kemiklioglu E, Atik E. The effect of the monomer functionality on the mechanical performance and polymer morphology of polymer stabilized blue phases. Composites Part B: Engineering. 2019 May 15;165:96-101.
- De Gennes PG. Scaling concepts in polymer physics: Ithaca, N.Y.: Cornell University Press, 1979.
- Sarva SS, Hsieh AJ.The effect of microstructure on the rate-dependent stress–strain behavior of poly(urethane urea) elastomers. Polymer 50(13):3007-301 June 2009.
- Efentakis, M., Al- hmoud, H., Choulis, N.H. Effect of additives on Fluorbiprofen controlled release preparation, Acta. Pharm. Technol. 1990; 36: 237- 239.
- Versypt AN, Pack DW, Braatz RD. Mathematical modeling of drug delivery from autocatalytically degradable PLGA Microspheres. Journal of Controlled Release, 2013; 165: 29–3
- Edlund U, Albertsson AC. Degradable polymer microspheres for controlled drug delivery. InDegradable aliphatic polyesters 2002 (pp. 67-112). Springer, Berlin, Heidelberg.
- Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. Journal of controlled release. 2003 Jul 31;90(3):261-80.
- Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. International journal of pharmaceutics. 2004 Sep 10;282(1-2):1-8.
- Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert opinion on biological therapy. 2004 Jan 1;4(1):35-51.
- Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996 Jan 1;17(2):103-14.
- Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced drug delivery reviews. 2012 Dec 1;64:72-82.
- Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. Journal of pharmacy and pharmacology. 2005 May;57(5):533-46.
- Dow T. Using methocel cellulose ethers for controlled release of drugs in hydrophilic matrix systems. Midland, MI: The Dow Chemical Company. 2000.
- Pygall SR, Whetstone J, Timmins P, Melia CD. Pharmaceutical applications of confocal laser scanning microscopy: The physical characterisation of pharmaceutical systems. Advanced Drug Delivery Reviews. 2007 Dec 10;59(14):1434-52.
- Hamed R, AlJanabi R, Sunoqrot S, Abbas A. The effect of pH, buffer capacity and ionic strength on quetiapine fumarate release from matrix tablets prepared using two different polymeric blends. Drug Dev Ind Pharm. 2017 Aug;43(8):1330-1342.
- Murari K, Gupta A, Dubey A K, Singh A, Mishra U K. Development of Sustained Release Floating Tablet for Cefpodoxime Proxetil (CP). Int. J. Pharm. Phytopharm. Res. 2019; 9(2): 96-105
- Vishnuvardhan K, Kavitha B, Nimmanapalli Y. Synthesis of silver nanoparticles using Aeschynomene indica L. aqueous leaf extract and evaluation of its Antibacterial activity. J. Biochem. Tech. 2020; 11(1): 1-13.
- Hosseini S M, Movahedi M, Majd A, Shafiee M, Ardestani S H. Evaluation of the Release of Nanoconjugate Recombinant Streptokinase Using State-Ease Software Application. J. Biochem. Tech. 2018; Special Issue (2): 121-128.
- Arti M, Ashwini A. Formulation and Evaluation of NitazoxanideSustained-Release Matrix Tablets. Int. J. Pharm. Phytopharm. Res. 2019; 9(3): 153-161.
- Hamed R, Physiological parameters of the gastrointestinal fluid impact the dissolution behavior of the BCS class IIa drug valsartan. Pharm. Dev. Technol. 2018 Dec;23(10):1168-1176.
- Ward A, Walton K, Mawla N, Kaialy W, Liu L, Timmins P, Conway BR, Asare-Addo K. Development of a novel method utilising dissolution imaging for the measurement of swelling behaviour in hydrophilic matrices. International journal of pharmaceutics: X. 2019 Dec 1;1:100013.
- Leong KW, Langer R. Polymeric controlled drug delivery. Advanced Drug Delivery Reviews. 1988 Sep 1;1(3):199-233.
- 21 - D.G. Kanjickal, S.T. Lopina, Modeling of drug release from polymeric delivery systems — a review, Crit. Rev. Ther. Drug Carrier Syst. 21 (2004) 345–386.
- Reddy KR, Mutalik S, Reddy S. Once-daily sustained-release matrix tablets of nicorandil: formulation and in vitro evaluation. AAPS pharmscitech. 2003 Dec 1;4(4):480-8.
- Mohammed AD., James LF, Michael HR., John EH., Rajabi Siahboomi AR. Release of propranolol hydrochloride from matrix tablets containing sodium carboxymethylcellulose and Hydroxypropyl methyl cellulose. Phar. Dev. Tech.,1999; 4: 313-324.
- Lee BJ, Ryu SG, Cui JH. Formulation and release characteristics of hydroxypropyl methylcellulose matrix tablet containing melatonin. Drug development and industrial pharmacy. 1999 Jan 1;25(4):493-501.
- Hassan AA, Dieaa HA. The Effect of Polymers Composition on the Release of Drug From Controlled Release Tablets. Lat. Am. J. Pharm. 2019;38(2):323-8.
- Al-Hmoud H, Efentakis M, Choulis NH. A controlled release matrix using a mixture of hydrophilic and hydrophobic polymers. International journal of pharmaceutics. 1991 Feb 1;68(1-3):R1-3.
- Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert opinion on drug delivery. 2010 Apr 1;7(4):429-44.
- Efentakis M, Al-Hmoud H, Buckton G, Rajan Z. The influence of surfactants on drug release from a hydrophobic matrix. International journal of pharmaceutics. 1991 Mar 31;70(1-2):153-8.
- Buckton G, Efentakis M, Al-Hmoud H, Rajan Z. The influence of surfactants on drug release from acrylic matrices. International journal of pharmaceutics. 1991 Aug 16;74(2-3):169-74.
- Efentakis M, Buckton G, Al-Hmoud H. The effect of surfactant charge on drug release from acrylic matrices. STP pharma sciences. 1992;2(4):332-6.
- Al-Hmoud HA, Ibrahim NE, El-Hallous EI. Surfactants solubility, concentration and the other formulations effects on the drug release rate from a controlled-release matrix. African Journal of Pharmacy and Pharmacology. 2014 Apr 8;8(13):364-71.
- Alhmoud HA, El-Hallous EI, Ibrahim NE, Attia AO, Dessoky ES. Formulation of propranolol hydrochloride controlled release tablets: Effect of surfactant charge and mechanisms of drug release. African Journal of Pharmacy and Pharmacology. 2014 Nov 22;8(43):1110-7.
- Alhmoud HA, Akkam YH. Combination of surfactants with other excipients: Effects on drug release and dimensional changes in matrices. Trop J Pharm Res 2019; 18(11):2241-2246.
- Cho K, Wang XU, Nie S, Shin DM. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin Cancer Res March 1, 2008 14:1310-1316.
- Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annual review of chemical and biomolecular engineering. 2010 Jul 15;1:149-73.
- Alhmoud H A. The effect of surfactant above and below the critical micelle concentration (CMC) and the mathematical models used to determine the kinetics of drug release from the matrix system. 2016; 10(8): 88-94.
- Frank S., lauterber P. C., Voltage-sensitive magnetic gels and magnetic resonance monitoring agents. Nature, 1993; 363: 334 – 336.
- Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annual review of chemical and biomolecular engineering. 2010 Jul 15;1:149-73.
- Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Advanced drug delivery reviews. 2007 Aug 10;59(8):718-28.
- Rawat A, Vaidya B, Khatri K, Goyal AK, Gupta PN, Mahor S, Paliwal R, Rai S, Vyas SP. Targeted intracellular delivery of therapeutics: an overview. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2007 Sep 1;62(9):643-58.
- Verma RK, Mishra B, Garg S. Osmotically controlled oral drug delivery. Drug Dev. Ind. Pharm. 2000;26(7):695–708.
- Langer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials; 1981;2(4):201–14.
- Berkland C, Pollauf E, Raman C, Silverman R, Kim KK, Pack DW. Macromolecule release from monodisperse PLG microspheres: control of release rates and investigation of release mechanism. Journal of pharmaceutical sciences. 2007 May 1;96(5):1176-91.
- Allison SD. Analysis of initial burst in PLGA microparticles. Expert opinion on drug delivery. 2008 Jun 1;5(6):615-28.
- Rothstein SN, Little SR. A “tool box” for rational design of degradable controlled release formulations. Journal of Materials Chemistry. 2011;21(1):29-39.
- Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—a review. International journal of pharmaceutics. 2011 Aug 30;415(1-2):34-52.
- Fredenberg S, Jönsson M, Laakso T, Wahlgren M, Reslow M, Axelsson A. Development of mass transport resistance in poly (lactide-co-glycolide) films and particles–A mechanistic study. International journal of pharmaceutics. 2011 May 16;409(1-2):194-202.
- Zhang L, Long C, Pan J, Qian Y. A Dissolution‐Diffusion Model and Quantitative Analysis of Drug Controlled Release from Biodegradable Polymer Microspheres. The Canadian Journal of Chemical Engineering. 2006 Oct;84(5):558-66.
- Perale G, Arosio P, Moscatelli D, Barri V, Müller M, MacCagnan S, Masi M. A new model of resorbable device degradation and drug release: Transient 1-dimension diffusional model. Journal of controlled release. 2009 Jun 19;136(3):196-205.
- Siepmann J, Peppas NA. Preface: Mathematical modeling of controlled drug delivery. Adv Drug Deliv Rev. 2001;48:137–138.
- Artifin DY, Lee LY, Wang CH. Mathematical modeling and simulation of drug release from microspheres: implication to drug delivery systems. Adv Drug Deliv Rev. 2006;58:1274–1325.
- Siepmann J, Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev. 2001;48:229–247.
- Narasimhan B. Mathematical models describing polymer dissolution: consequences for drug delivery. Adv Drug Deliv Rev. 2001;48:195–210.
- Vidyadhara S, Rao PR, Prasad JA. Formulation and evaluation of propranolol hydrochloride oral controlled release matrix tablets. Indian journal of pharmaceutical sciences. 2004;66(2):188.
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. International journal of pharmaceutics. 1983 May 1;15(1):25-35.
- Ritger PL, Peppas NA. A simple equation for description of solute release. I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 1987; 5(1):23–36.
- Ritger PL, Peppas NA. A simple equation for description of solute release; II. Fickian and anomalous release from swellable devices. J. Control Release. 1987; 5(1):37– 42.
- Siepmann J, Siepmann F. Modeling of diffusion controlled drug delivery. Journal of controlled release. 2012 Jul 20;161(2):351-
- Yin C, Li X. Anomalous diffusion of drug release from a slab matrix: Fractional diffusion models. International journal of pharmaceutics. 2011 Oct 10;418(1):78-87.
- Muffly TM, Tizzano AP, Walters MD. The history and evolution of sutures in pelvic surgery. Journal of the royal society of medicine. 2011 Mar 1;104(3):107-12.
- Stamatialis DF, Papenburg BJ, Girones M, Saiful S, Bettahalli SN, Schmitmeier S, Wessling M. Medical applications of membranes: drug delivery, artificial organs and tissue engineering. Journal of Membrane Science. 2008 Feb 1;308(1-2):1-34.
- Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Progress in polymer science. 2007 Aug 1;32(8-9):762-98.
- Tian H, Tang Z, Zhuang X, Chen X, Jing X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Progress in Polymer Science. 2012 Feb 1;37(2):237-80.
- Bat E, Zhang Z, Feijen J, Grijpma DW, Poot AA. Biodegradable elastomers for biomedical applications and regenerative medicine. Regenerative medicine. 2014 May;9(3):385-98.
- Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjugate chemistry. 1995 Jul 1;6(4):332-51.
- Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: a unique polymer for drug delivery. Therapeutic delivery. 2015 Jan;6(1):41-58.
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nature reviews Drug discovery. 2010 Aug;9(8):615-27.
- Huang X, Voit B. Progress on multi-compartment polymeric capsules. Polymer Chemistry. 2013;4(3):435-43.
- Mohamed S, Parayath NN, Taurin S, Greish K. Polymeric nano-micelles: versatile platform for targeted delivery in cancer. Therapeutic delivery. 2014 Oct;5(10):1101-21.
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]