Preparation and physicochemical characterization of cocrystals for enhancing the dissolution rate of glimepiride
Iman S Jaafar*, Ameera A Radhi
Department of Pharmaceutics, College of Pharmacy, University of Mustansiriyah, Baghdad, Iraq.
Correspondence: Iman S Jaafar, Department of Pharmaceutics, College of Pharmacy, University of Mustansiriyah, Baghdad, Iraq.
ABSTRACT
Aim: Glimepiride (Glmp) is an oral hypoglycemic drug used for the treatment of type 2 diabetes mellitus. It has low and unpredictable bioavailability that is mainly caused by its poor aqueous solubility and low dissolution rate. Accordingly, the objective of the present study was to improve the dissolution rate of Glmp by cocrystallization with suitable water-soluble coformers. Methods: Glmp was screened with three potential coformers, namely citric acid, tartaric acid, and oxalic acid dihydrate. Formulations were prepared at Glmp: coformer molar ratio of (1:1) and (1:2) using the solvent evaporation method. The obtained products were then characterized by differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and in vitro dissolution studies. Results and discussion: Data obtained from DSC, PXRD, and FTIR spectra suggested cocrystal formation. Cocrystals also achieved a remarkably higher dissolution rate than the parent drug. F4 containing Glmp: tartaric acid (1:2) was selected as the optimum formulation. Conclusion: In the present study, Glimepiride cocrystals are introduced as a promising strategy for a possible transition to a suitable drug product.
Keywords: Bioavailability, crystal engineering, coformer, solubility.
Introduction
Solubility is a very important characteristic of drugs [1, 2]; it mainly influences the efficacy of drugs in biological fluids [3, 4]. A wide range of newly invented active ingredients in the pharmaceutical industry is of poor aqueous solubility. This poor solubility results in deprived and variable bioavailability. Concerning the orally administered dosage form, poor solubility is the main parameter that limits the drug absorption to the systemic circulation, consequently prevents the attainment of the required concentration for therapeutic activity. [5, 6] Different techniques are available to improve the solubility of poorly aqueous soluble drugs. [7] These techniques include a reduction in particle size, salt formation, microemulsion, nanocrystals, solid dispersions, inclusion complexes, amorphous dispersions, and cocrystals. [8, 9]
Pharmaceutical cocrystal can be defined as a multi-component crystalline solid that consists of two (or more) neutral molecules held together via non-covalent interactions at a specific stoichiometric ratio. Cocrystals mainly consist of the active pharmaceutical ingredients (APIs) and a coformer, which may be either an excipient or a different drug. [10]
Cocrystals have emerged as a promising tool in improving the solubility and dissolution profile of poorly aqueous soluble drugs. [11] Despite the fact that salt formation represents the simplest and the most commonly used method in increasing solubility of many APIs, its use is limited only to ionizable compounds. In contrast, both ionizable and non-ionizable APIs can form cocrystals. [12]
Glmp is an oral hypoglycemic drug used for the treatment of type 2 diabetes mellitus which belongs to third-generation sulfonylurea agents. It acts by stimulating the functioning of pancreatic beta cells to release insulin and increase peripheral tissue insulin sensitivity. [13, 14] Glmp is a drug with poor aqueous solubility and high permeability which merits it to be categorized under class II drugs in the biopharmaceutical classification system (BCS). This poor solubility and low dissolution rate results in low and unpredictable bioavailability. [15]
Various novel pharmaceutical technologies, such as solid dispersions, nanocrystal, micelles, and self-nanoemulsifying systems [16] have been used to address the solubility problem of Glmp. This study attempted at preparing Glmp cocrystals and investigated the impact of type and molar ratio of coformers on the dissolution pattern of Glmp from these formulations.
Material and Methods
Glimepiride (Glmp) was purchased from (Lupio medico, India). The citric acid (CA) and tartaric acid (TA) were purchased from (Panreac Quimica S.L.U., Barcelona). Oxalic acid (OA) and sodium lauryl sulfate (SLS) were purchased from Fluka AG (Switzerland).
Preparation of Glmp cocrystals
Six formulations containing Glmp: coformer in molar ratios of 1:1 and 1:2, as shown in Table 1, were dissolved in 100 ml of methanol and transferred to a round bottom flask of the rotary evaporator (Buchi, Germany). Then, methanol was allowed to evaporate at 55 ⁰C and reduced pressure (100 mbar) and rotation speed at 100 revolutions per minute (rpm).
After evaporation of methanol, the vacuum was decreased to 50 mbar and held for 10 min to allow complete removal of the residual solvent. The resultant formulations were sieved, then kept in desiccators for further investigation.
|
Table 1: Physical mixture of Glmp cocrystals |
||||
|
Ingredient Formulation |
Glmp (mg) |
CA (mg) |
TA (mg) |
OA (mg) |
|
F1 (1:1) |
100 |
38 |
|
|
|
F2 (1:2) |
100 |
76 |
|
|
|
F3 (1:1) |
100 |
|
30 |
|
|
F4 (1:2) |
100 |
|
60 |
|
|
F5 (1:1) |
100 |
|
|
25.2 |
|
F6 (1:2) |
100 |
|
|
50.4 |
Physicochemical characterization of the prepared cocrystals
Differential scanning calorimetry (DSC)
A differential scanning calorimeter (SAT PT-1000 Linseis) was used to carry out thermal analysis on unprocessed Glmp, coformers, and the Glmp cocrystal samples. A known amount of each powder sample was placed in aluminum pans which were hermetically sealed. The thermal analysis was carried out using a heating rate of 10°C/min covering a heating range of 0-400 °C. The scanning procedure was conducted under argon atmosphere.
Powder X-ray diffraction (PXRD)
The diffraction patterns for unprocessed drug, coformers and the Glmp cocrystal samples were obtained using Shimadzu X ray diffractometer (XRD 6000) equipped with Cu radiation source. The X-ray tube was set at a voltage of 40.0 kV and a current of 30 mA. The measurements were carried out at ambient temperature over 2ϴ drive axis, the scan range of 5000-80000 (degree), and continuous scanning mode.
Dissolution studies
The dissolution studies of the unprocessed drug and cocrystals were employed using the USP Type II dissolution apparatus containing 900 ml phosphate buffer (pH 6.8) containing 1.5% sodium lauryl sulfate maintained at 37±1oC and stirring rate of 50 rpm. Samples corresponding to 10 mg of Glmp were added into the dissolution vessels. Aliquots (5 ml) were withdrawn periodically and replenished each time with 5ml fresh medium to maintain the concentration gradient. The concentration of the dissolved drug was determined spectrophotometrically at a wavelength of 229 nm. The dissolution studies were conducted in triplicates.
Fourier transform infrared spectroscopy (FTIR)
FT-IR spectra were recorded using the FTIR spectrophotometer (Shimadzu FTIR-8400S). Potassium bromide (spectroscopic grade) was mixed with dry samples prior to compression to disks using a hydraulic press. The scans were run in the wavelength region of 400–4000 cm−1.
Results and Discussion
Differential scanning calorimetry (DSC)
Data obtained from DSC thermograms are presented in table (2). The thermogram of unprocessed Glmp shows a sharp endothermic peak at 212.5°C, corresponding to its melting point.[17] The DSC curves of the prepared formulations showed both the absence of peaks that correspond to either the pure drug or the coformers and significant changes in enthalpy suggesting the cocrystals formation. [18, 19]
All the prepared formulations, except F2 and F6, exhibited lower melting temperature (Tm) than both Glmp and the coformer. It has been reported in the literature that Tm of cocrystals might occur between the melting peaks of the parent drug and the coformer or reduced below that of both components. Reduced melting events have a positive influence on the solubility of the product. [20]
The appearance of higher endothermic peaks in F2 and F6 is less frequently seen in cocrystals. However, it has been observed in other studies as in dexlansoprazole cocrystals. [21] DSC curves of Glmp, tartaric acid, and F4 are depicted in figures 1, 2, and 3 respectively.
|
Table 2: Thermal analysis data for Glmp, coformers and the prepared formulations |
||||
|
Sample |
Onset (°C) |
Offset (°C) |
Tm (°C) |
Enthalpy(J/g) |
|
Glmp |
208.2 |
219.3 |
212.5 |
-144.99 |
|
Citric acid |
151.9 |
169.5 |
160.3 |
-160.34 |
|
187.8 |
239.9 |
221.9 |
-342.35 |
|
|
F1 Glmp: citric acid (1:1) |
130.5 |
167.0 |
150.8 |
-100.82 |
|
F2Glmp: citric acid (1:2) |
133.6 |
161.8 |
149.7 |
-78.00 |
|
247.5 |
285.4 |
268.7 |
-70.32 |
|
|
Tartaric acid |
207.5 |
226.2 |
215.2 |
-468.2 |
|
F3 Glmp: tartaric acid (1:1) |
67.7 |
98.0 |
82.1 |
-39.71 |
|
152.5 |
179.2 |
166.7 |
-77.08 |
|
|
F4 Glmp: tartaric acid (1:2) |
67.0 |
105.2 |
84.3 |
-63.45 |
|
147.5 |
171.9 |
164.4 |
-77.05 |
|
|
Oxalic acid |
77.1 |
143.1 |
119.9 |
-569.12 |
|
190.6 |
210.1 |
199.2 |
-414.47 |
|
|
F5 Glmp: oxalic acid (1:1) |
51.2 |
94.2 |
74.0 |
-56.25 |
|
115.5 |
134.9 |
125.4 |
-12.16 |
|
|
F6 Glmp: oxalic acid (1:2) |
57.6 |
107.1 |
87.7 |
-103.25 |
|
119.3 |
144.3 |
131.4 |
-44.55 |
|
|
192.1 |
235.5 |
219.3 |
-102.31 |
|
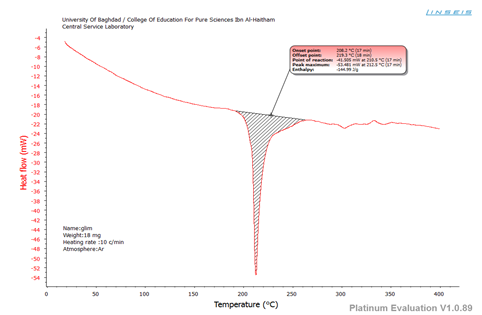
Figure 1: DSC curve of GLMP
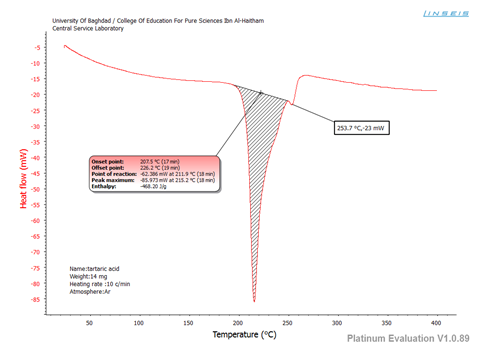
Figure 2: DSC curve of tartaric acid
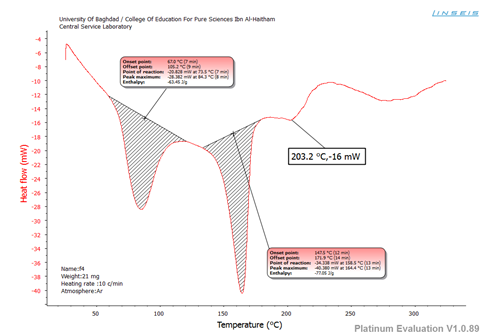
Figure 3: DSC curve of F4
Powder X-ray diffraction (PXRD)
Glmp showed characteristic diffraction peaks at 2θ 13.47°, 16.7°, 18.16°, 19.18°, and 21.09° (figure 4) indicating its crystalline nature. [16] The crystalline characteristics for citric acid, tartaric acid, and oxalic acid dihydrate were also demonstrated in the diffractograms.
The appearance of additional peaks, not related to either the pure drug or the coformer, in the spectra of all formulations (F1-F6) clearly implies the formation of a new crystalline phase. [22] This has been observed in X-ray spectrum of the optimum formulation F4, depicted in figure 6, as it showed unique peaks when compared to the diffraction pattern of GLMP or tartaric acid (figure 5).
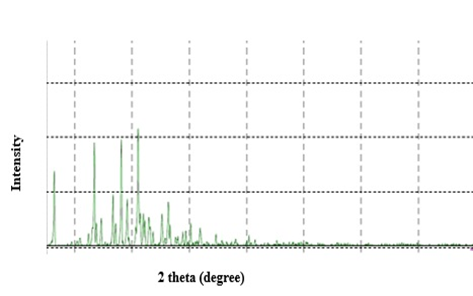
Figure 4: X-ray spectra of Glmp
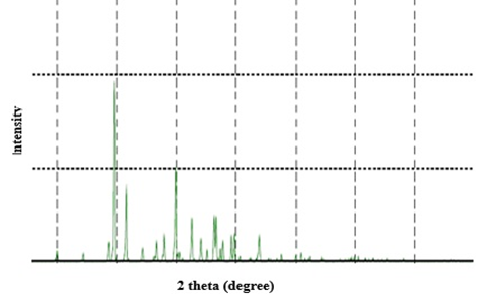
Figure 5: X-ray spectra of tartaric acid
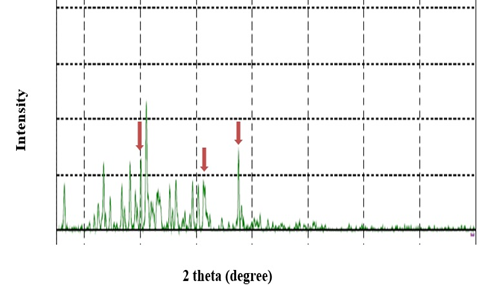
Figure 6: X-ray spectra of F4
Dissolution studies
The dissolution profiles of the unprocessed Glmp and the corresponding cocrystals prepared using citric acid; tartaric acid and oxalic acid dihydrate are depicted in figures 7, 8, and 9 respectively. The results clearly revealed that cocrystals have markedly improved the dissolution pattern of Glmp. The remarkable increase in the dissolution rate could be explained by the modification in the crystal habit where molecules might be arranged in less packed crystalline arrays via weaker intermolecular bonding compared to the parent drug molecules. This explanation is strongly supported by the data obtained from DSC and PXRD implying the production of new crystalline states. [23]
A higher dissolution rate also might be attributed to the fact that the coformers used in the study are highly soluble in the aqueous medium, which leads to dissociation of the cocrystals to its components and thereby allowing fast dissolution. [24]
To investigate the impact of the coformer ratio on the dissolution patterns, cocrystals were prepared at molar ratios 1:1 and 1:2 with all coformers used in the study.
F1 (1:1) and F2 (1:2) containing citric acid liberated 67.59 and 81.01 percent after 45 minutes, respectively. It could be inferred that increasing the quantity of citric acid has achieved a significant impact on the dissolution rate, and thus indicates co-crystal formation at a molar ratio of 1:2. [25] Similarly, tartaric acid quantity had a significant influence on the dissolution rate. F4 (1:2) was superior compared to F3 (1:1) by which it liberated 99.92 percent of the labeled amount in only 5 minutes while F3 released 63.35 percent at the end of 60 minutes. Therefore, cocrystals formation at 1:2 stoichiometry could be considered. [24]
A reduction in the release rate was observed after increasing the molar ratio of oxalic acid dihydrate from 1:1 in F5 into 1:2 in F6. This finding could be attributed to a modification in the crystal lattice causing an increase in the density of the cocrystals, thereby lowering the dissolution rate of Glmp from cocrystals. [26, 27] Based on the above results, F4 was selected as the optimum formulation as it exhibited the fastest release rate.
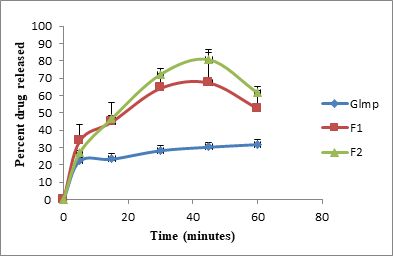
Figure 7: In vitro dissolution behavior of Glmp, F1, and F2
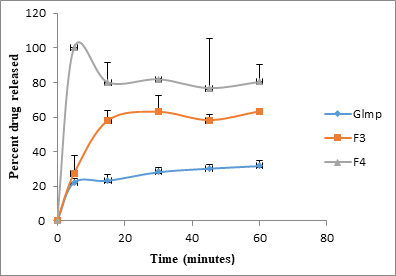
Figure 8: In vitro dissolution behavior of Glmp, F3, and F4
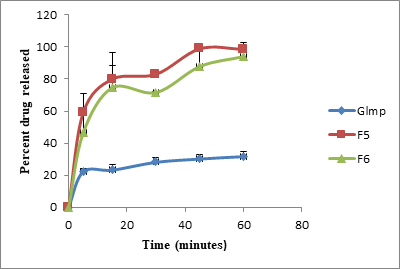
Figure 9: In vitro dissolution behavior of Glmp, F5, and F6
Fourier transform infrared spectroscopy (FTIR)
Cocrystals are multicomponent assemblies stabilized primarily through hydrogen bonding. FTIR spectroscopy has been implemented to detect these interactions based on observation of significant alterations in the absorption spectra compared to the original spectra for the parent compounds. The spectrum of Glmp exhibited peaks at 3369.75 and 3288.74 cm-1 assigned to NH stretching bands for urea, peaks at 1350.22 and 1159.26cm-1 corresponding to the sulfonamide (S=O) group and peaks at 1707.06 and 1672.34 cm-1 corresponding to the carbonyl group (C=O). This spectrum is consistent with those reported in the literature. [16]
The spectra of citric acid, tartaric acid, and oxalic acid dihydrate exhibited the characteristics peaks corresponding to C=O stretching vibration occurring in the region (1760-1690) cm-1 and the broadband assigned to carboxylic acid hydroxyl group in the region (3300-2500) cm-1. [28] The spectra of cocrystals formulations F1-F6 revealed prominent changes in peaks corresponding to S=O stretching vibration of Glmp as alterations in frequencies (table 3). A similar observation has been recorded for peaks assigned to C=O of the acid. Besides, the decreased intensity and the recorded changes in the shape of the absorption band corresponding to the hydroxyl group of carboxylic acids suggest hydrogen bonds formed between the hydroxyl group of acid with S=O group in Glmp. [29] Figures 10, 11, and 12 depict the FTIR spectra of Glmp, tartaric acid, and the optimum formulation F4.
|
Table 3. Characteristic FTIR peaks for Glmp, coformers and the prepared formulations |
|||||
|
|
Characteristic peaks and positions (cm-1) |
||||
|
Sample |
NH-stretching |
Glmp C=O stretching |
Coformer C=O stretching |
S=O stretching |
OH stretching |
|
Glmp |
3369.75 3288.74 |
1672.34 1707.06 |
----- |
1350.22 1159.26 |
----- |
|
Citric acid |
--------- |
----- |
1751.42
|
----- |
2785.3-3497.06 |
|
F1 Glmp : citric acid (1:1) |
3369.75 3288.74 |
1672.2 1707.06 |
1737.92 |
1348.29 1155.4 |
Decreased intensity and changed shape |
|
F2 Glmp: citric acid (1:2) |
3288.74 |
1672.2 1707.06 |
1753.35 |
1350.22 1149.61 |
Decreased intensity and changed shape |
|
Tartaric acid |
-------- |
------ |
1749.49 |
------ |
2650.28-3410.26 |
|
F3 Glmp: tartaric acid (1:1) |
3367.82 3288.74 |
1674.27 1707.06 |
1739.85 |
1348.29 1153.47 |
Decreased intensity and changed shape |
|
F4 Glmp: tartaric acid (1:2) |
3365.9 3290.67 |
1670.41 1707.06 |
1743.71 |
1348.29 1153.47 |
Decreased intensity and changed shape |
|
Oxalic acid |
-------- |
------- |
1697.41 |
|
3427.62-3529.85 |
|
F5 Glmp: oxalic acid (1:1) |
3369.75 3288.74 |
1672.2 1708.99 |
Difficult to assign |
1348.29 1155.4 |
Decreased intensity and changed shape |
|
F6 Glmp: oxalic acid (1:2) |
3369.75 3290.67 |
1672.13 1707.06 |
Difficult to assign |
1348.29 1155.4 |
Decreased intensity and changed shape |
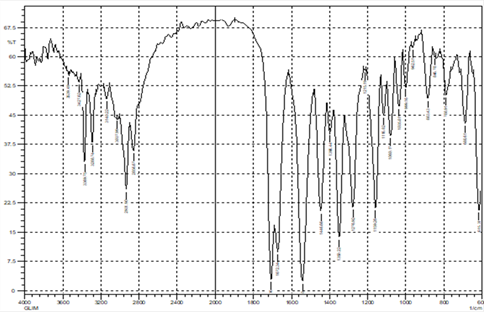
Figure 10: FTIR spectra of GLMP
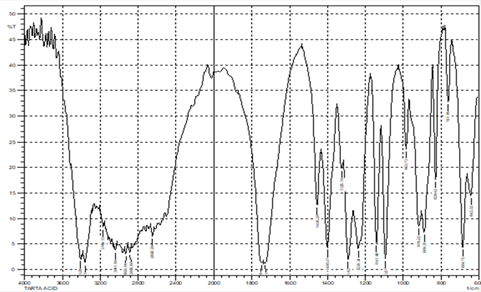
Figure 11: FTIR spectra of tartaric acid
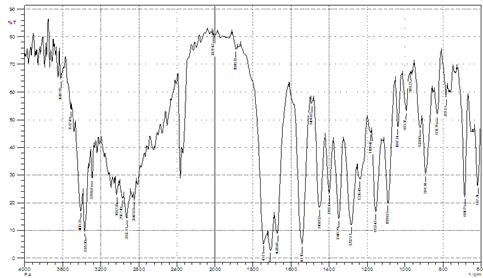
Figure 12: FTIR spectra of F4
Conclusion
In conclusion, the cocrystallization of glimepiride with water-soluble coformers might be considered as a promising approach with improved dissolution rate profile. Further in vivo analysis for the selected formula is recommended so that it may extend into a suitable drug product.
Acknowledgment
The authors are extremely grateful for the provision from the University of Mustansiriyah (www. uomustansiriyah.edu.iq), Baghdad, Iraq.
References
- Nasser N H, Hussein S A, Hussein A K, Alibeg A A, Jasim Z M. Design, Synthesis, and Antibacterial Study of New Gatifloxacin-Antioxidants as Mutual Prodrugs. J. Biochem. Tech. 2020; 11(1): 32-36.
- Bahreini M, Movahedi M, Peyvandi M, Nematollahi F, Tehrani H S. Assessment of cytotoxicity and artificial peroxidase activity of multi wall Carbon Nano tubes. J. Biochem. Tech. 2018; 9(2): 7-9.
- Jafar M, Alshaer F I, Adrees F A. Cyclodextrin Ternary Inclusion Complexation: A Strategy to Improve Solubility of Poorly Soluble Drugs. Int. J. Pharm. Phytopharm. Res. 2018; 8(6): 8-17.
- Mhetre R L, Hol V B, Dhole S N. Design of Telmisartan Loaded Nanoparticles by Three Square Factorial Design Approach. Int. J. Pharm. Phytopharm. Res. 2018; 8(4): 53-62.
- Iman S, Jaafar MHS, Mahmood SZ. Formulation and In-vitro Evaluation of Fast Dissolving Tablets of Meloxicam Solid Dispersion. Int J Pharm Sci Rev Res. 2016;41(1):
- Aldahhan ES. Co-Amorphous System: A promising Strategy for Delivering Poorly Water-Soluble Drugs. IJPS. 2020;29(1):1-11.
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN pharmaceutics. 2012;2012.
- Jabar EG, Taghi HS, Hasson KJ, Alkhalifa II. Evaluation of innovated formula of Bisacodyl suppository following the dissolution profile and stability data by using developed HPLC method. JAPER. 2020;10(2).
- Ali J, Zgair A, Hameed GS, Garnett MC, Roberts CJ, Burley JC, Gershkovich P. Application of biorelevant saliva-based dissolution for optimisation of orally disintegrating formulations of felodipine. Int J Pharm. 2019;555:228-36.
- Remenar JF, Morissette SL, Peterson ML, Moulton B, MacPhee JM, Guzmán HR, Almarsson Ö. Crystal engineering of novel cocrystals of a triazole drug with 1, 4-dicarboxylic acids. J Am Chem Soc. 2003;125(28):8456-7.
- Apshingekar PP, Aher S, Kelly AL, Brown EC, Paradkar A. Synthesis of caffeine/maleic acid co-crystal by ultrasound-assisted slurry co-crystallization. J Pharm Sci. 2017;106(1):66-70.
- Basavoju S, Boström D, Velaga SP. Indomethacin–saccharin cocrystal: design, synthesis and preliminary pharmaceutical characterization. Pharm Res. 2008;25(3):530-41.
- Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D’Alessio DA, Davies MJ. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487-93.
- Park H, Seo HJ, Ha ES, Hong SH, Kim JS, Kim MS, Hwang SJ. Preparation and characterization of glimepiride eutectic mixture with L-arginine for improvement of dissolution rate. Int J Pharm. 2020:119288.
- Medarević D, Ibrić S, Vardaka E, Mitrić M, Nikolakakis I, Kachrimanis K. Insight into the formation of glimepiride nanocrystals by wet media milling. Pharmaceutics. 2020;12(1):53.
- Li H, Ma L, Li X, Cui X, Yang W, Shen S, et al. A simple and effective method to improve bioavailability of glimepiride by utilizing hydrotropy technique. Eur J Pharm Sci. 2015;77:154-60.
- Mohamed EA, Meshali MM, Foda AMM, Borg TM. Improvement of dissolution and hypoglycemic efficacy of glimepiride by different carriers. AAPS PharmSciTech. 2012;13(3):1013-23.
- Arafa MF, El-Gizawy SA, Osman MA, El Maghraby GM. Co-crystallization for enhanced dissolution rate of nateglinide: In vitro and in vivo evaluation. J Drug Deliv Sci Tech. 2017;38:9-17.
- Machado TC, Gelain AB, Rosa J, Cardoso SG, Caon T. Cocrystallization as a novel approach to enhance the transdermal administration of meloxicam. Eur J Pharm Sci. 2018;123:184-90.
- Qiao N, Li M, Schlindwein W, Malek N, Davies A, Trappitt G. Pharmaceutical cocrystals: an overview. Int J Pharm. 2011;419(1-2):1-11.
- Li J, Wang L, Ye YQ, Fu X, Ren Q, Zhang H, Deng Z. Improving the solubility of dexlansoprazole by cocrystallization with isonicotinamide. Eur J Pharm Sci. 2016;85:47-52.
- Nijhawan M, Santhosh A, Babu PS, Subrahmanyam C. Solid state manipulation of lornoxicam for cocrystals–physicochemical characterization. Drug Dev Ind Pharm. 2014;40(9):1163-72.
- Bethune SJ, Huang N, Jayasankar A, Rodríguez-Hornedo Nr. Understanding and predicting the effect of cocrystal components and pH on cocrystal solubility. Crystal Growth & Design. 2009;9(9):3976-88.
- Arafa MF, El-Gizawy SA, Osman MA, El Maghraby GM. Xylitol as a potential co-crystal co-former for enhancing dissolution rate of felodipine: preparation and evaluation of sublingual tablets. Pharm Dev Technol. 2018;23(5):454-63.
- El-Gizawy SA, Osman MA, Arafa MF, El Maghraby GM. Aerosil as a novel co-crystal co-former for improving the dissolution rate of hydrochlorothiazide. Int J Pharm. 2015;478(2):773-8.
- Goud NR, Khan RA, Nangia A. Modulating the solubility of sulfacetamide by means of cocrystals. CrystEngComm. 2014;16(26):5859-69.
- Kaur R, Cavanagh KL, Rodríguez-Hornedo N, Matzger AJ. Multidrug cocrystal of anticonvulsants: influence of strong intermolecular interactions on physiochemical properties. Crystal Growth & Design. 2017;17(10):5012-6.
- Fung MH, DeVault M, Kuwata KT, Suryanarayanan R. Drug-Excipient Interactions: Effect on Molecular Mobility and Physical Stability of Ketoconazole–Organic Acid Coamorphous Systems. Mol Pharm. 2018;15(3):1052-61.
- Arafa MF, El-Gizawy SA, Osman MA, El Maghraby GM. Sucralose as co-crystal co-former for hydrochlorothiazide: development of oral disintegrating tablets. Drug Dev Ind Pharm. 2016;42(8):1225-33.
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]