Novel biomaterial from mud-crab shell as alternative for maxillofacial trauma treatment
Shanti F. Boesoirie1, Putri Hartini1, Raden A. Kartiwa1, Mohamad R. Dahlan1, Bambang Setiohadjie1, Arif S. W. Kusuma2,3*, Nur Atik4, Basril Abbas5, Paramita Pandansari5
1Department of Ophthalmology, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia. 2Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia. 3Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia. 4Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia. 5National Nuclear Energy Agency of Indonesia, Jakarta 4000, Indonesia (Badan Tenaga Nuklir Nasional/BATAN).
ABSTRACT
Titanium-based material or commonly known as “titanium plate” is a type of implant that is widely used for maxillofacial trauma treatment. The use of titanium plate has shown a great impact on osteo-conductivity but it is still lacking an impact on osteo-stimulation promotion. Mud crab shell has components that are very similar to human bone. Thus, the use of mud crab shell as a novel material is expected to mimic the osteo-conductive, osteo-stimulation/ and osteo-integration ability of natural human bone. The compatibility of the mud crab shell as a biomaterial can be assessed by testing the foreign body giant cells. This was an animal-model study with the implantation of titanium plates and crab shell. Fourteen Wistar strain of white male rats (Rattus norvegicus) were randomly divided into two different groups. Injury in the calvaria bone was applied to both groups. Then, the first group was treated with titanium plate implantation while the second group was treated with mud crab shell implantation. Fifteen days after the implantation, the calvaria bone from both groups was extracted and histologic preparation was made. The counting procedure of foreign body giant cells and inflammatory-infiltration number were performed by using a microscope. The number of foreign body giant cells found in the first group was lower compared to the second group, while the inflammatory-infiltration of both groups was found to be as equal. Biomaterial from mud crab (Scylla serrata) shell is potential as a novel xenograft material for maxillofacial trauma treatment.
Keywords: mud crab shell, implant, calvaria bone, foreign body giant cell.
Introduction
Maxillofacial trauma is a trauma related to the damage of facial bones and its adjacent tissues [1]. In the United States alone, the incidence rate of this trauma to happen was 3 million cases per year with the main cause of vehicle accidents (approximately 40-50%) [2, 3]. Maxillofacial trauma that could directly affect the orbital bone fracture, which urgently needs a surgical intervention as a treatment. An implant over the fracture is often needed to prevent the recurrent adhesion and orbital tissue prolapse [4]. The ability to select the right type of implant remains one of the challenges that occur in maxillofacial trauma management. The implant used for the management of maxillofacial trauma were divided into autograft, allograft, autoplastic, and xenograft. [5-8] Whereas the drawback of using titanium plate material is the economical factor as it is too expensive. [6, 8-12]
A pilot study as an attempt to search for novel bone alternatives in maxillofacial surgery was conducted by Dupoirieux and Wilson et al. The study has found that there is a high similarity of human bone and mud crab shell in terms of microstructure. Both biomaterials were formed by a long-term biomineralization process and utilize calcium as its main component. Both human bones and mud crab shells also play an important role as a supporting structure for internal organs protection. The main difference was human bones are located inside the body while mud crab shells are located outside the body. Based on microscopic analysis, both human bones and mud crab shells possessed an ability to store various micronutrients such as calcium, phosphate, and also carbonate. Moreover, mud crab shells are quite easy to get based on its natural availability and also its simplicity to be processed as a biomaterial alternative for bone fracture implantation. [13-18] Thus, the aim of this study was to evaluate the mud crab shell biomaterial properties as an alternative to replace the titanium plate to be used on the orbital bone area.
Materials and Methods
Animal Model
This study used the Wistar strain of white male rats (Rattus norvegicus) as the animal model. Experimental protocols performed in this study comply with the guidelines of the Animal Care and Research Ethics Committee from Universitas Padjadjaran. A total of fourteen healthy and active male rats aged four months (weigh 250-300 grams) were used in this study with initial acclimatization for approximately seven days.
During the acclimatization period, all animals included were observed and animals with abnormalities on both calvaria bone and/or did not look too active (sick) were excluded from the study. Animals were then housed randomly into two wooden stainless cages, each cage with seven rats under controlled temperature, humidity, ventilation, and also a 12-hour light-dark cycle. Chows and water were provided ad libitum. Estimation of the sample number was count by Mead’s resource equation method.
After seven days, a similar type of artificial bone injury was performed into both groups. Each animal in the first group was then implanted with 10x4 mm of titanium plate (as standard), while second group animals were implanted with 10x10 mm of sterilized mud crab shell biomaterial.
Crab Shell and Titanium Plate
Mud-crab (Scylla serrata) shells used in this study were originated from a local crabs breeding facility. Inside parts of mud-crab shells were extracted by using forceps and shaped into a square plate with a size of 10x10 mm by using 2 mm drill equipment. Processed shells were then taken into the Indonesian National Agency of Nuclear Energy (Badan Tenaga Nuklir Nasional/BATAN) for further preparation steps. The major preparation steps including: (1) deproteinization; (2) lyophilization; and (3) sterilization.
Details included in this procedure were soaking the crab shells for an hour in 3% of H2O2 and demineralization using HCl 0.6 M also for an hour. The soaking process was then continued by using 2-propanolol for a day (24 hours). Another soaking procedure was performed for 72 hours (at 37°C) by using a mixture of 2 mM sodium azide and iodoacetic acid (with an additional phosphate buffer). Final products were then cleaned with distilled water and stored on -40°C for three days to prevent bacterial growth.
Introduction
Maxillofacial trauma is a trauma related to the damage of facial bones and its adjacent tissues [1]. In the United States alone, the incidence rate of this trauma to happen was 3 million cases per year with the main cause of vehicle accidents (approximately 40-50%) [2, 3]. Maxillofacial trauma that could directly affect the orbital bone fracture, which urgently needs a surgical intervention as a treatment. An implant over the fracture is often needed to prevent the recurrent adhesion and orbital tissue prolapse [4]. The ability to select the right type of implant remains one of the challenges that occur in maxillofacial trauma management. The implant used for the management of maxillofacial trauma were divided into autograft, allograft, autoplastic, and xenograft. [5-8] Whereas the drawback of using titanium plate material is the economical factor as it is too expensive. [6, 8-12]
A pilot study as an attempt to search for novel bone alternatives in maxillofacial surgery was conducted by Dupoirieux and Wilson et al. The study has found that there is a high similarity of human bone and mud crab shell in terms of microstructure. Both biomaterials were formed by a long-term biomineralization process and utilize calcium as its main component. Both human bones and mud crab shells also play an important role as a supporting structure for internal organs protection. The main difference was human bones are located inside the body while mud crab shells are located outside the body. Based on microscopic analysis, both human bones and mud crab shells possessed an ability to store various micronutrients such as calcium, phosphate, and also carbonate. Moreover, mud crab shells are quite easy to get based on its natural availability and also its simplicity to be processed as a biomaterial alternative for bone fracture implantation. [13-18] Thus, the aim of this study was to evaluate the mud crab shell biomaterial properties as an alternative to replace the titanium plate to be used on the orbital bone area.
Materials and Methods
Animal Model
This study used the Wistar strain of white male rats (Rattus norvegicus) as the animal model. Experimental protocols performed in this study comply with the guidelines of the Animal Care and Research Ethics Committee from Universitas Padjadjaran. A total of fourteen healthy and active male rats aged four months (weigh 250-300 grams) were used in this study with initial acclimatization for approximately seven days.
During the acclimatization period, all animals included were observed and animals with abnormalities on both calvaria bone and/or did not look too active (sick) were excluded from the study. Animals were then housed randomly into two wooden stainless cages, each cage with seven rats under controlled temperature, humidity, ventilation, and also a 12-hour light-dark cycle. Chows and water were provided ad libitum. Estimation of the sample number was count by Mead’s resource equation method.
After seven days, a similar type of artificial bone injury was performed into both groups. Each animal in the first group was then implanted with 10x4 mm of titanium plate (as standard), while second group animals were implanted with 10x10 mm of sterilized mud crab shell biomaterial.
Crab Shell and Titanium Plate
Mud-crab (Scylla serrata) shells used in this study were originated from a local crabs breeding facility. Inside parts of mud-crab shells were extracted by using forceps and shaped into a square plate with a size of 10x10 mm by using 2 mm drill equipment. Processed shells were then taken into the Indonesian National Agency of Nuclear Energy (Badan Tenaga Nuklir Nasional/BATAN) for further preparation steps. The major preparation steps including: (1) deproteinization; (2) lyophilization; and (3) sterilization.
Details included in this procedure were soaking the crab shells for an hour in 3% of H2O2 and demineralization using HCl 0.6 M also for an hour. The soaking process was then continued by using 2-propanolol for a day (24 hours). Another soaking procedure was performed for 72 hours (at 37°C) by using a mixture of 2 mM sodium azide and iodoacetic acid (with an additional phosphate buffer). Final products were then cleaned with distilled water and stored on -40°C for three days to prevent bacterial growth.
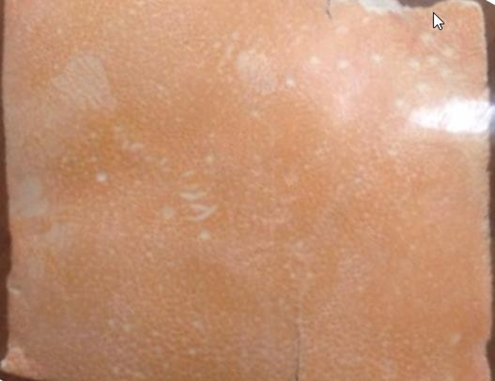
Figure 1. An image of mud-crab shells (in a plate form) final product
The titanium plates used in this study were the Osteomed Craniomaxillofacial CFXTM. The microplates have 18 holes with 1,2 mm diameter and were cut down into a 10x4 mm size per plate by using plate scissors.
Artificial calvarian bone injury
Male Wistar rats were anesthetized with an intraperitoneal dose of Ketamine hydrochloride (0,3 ml/100 gram of body weight) and Xylazine hydrochloride 2% (5-10 mg/Kg of body weight). Rats were then placed under a microscope for minor surgery and were given povidone-iodine (as an antiseptic) on the calvaria bone area. Two centimeters of linear-horizontal incision was made at the frontal area subcutis of each rat. Then, an incision at the periosteum part was made and continued with a dissection by using a dissector on the top part of calvaria bone. The artificial injury size of each rat was 10 mm and it was made by using a drill. The injury could be observed from the bleeding of intraosseous blood vessels.
For the test group, the mud-crab shell biomaterials were implanted under the periosteum, above the injured bone. While for the control group, titanium plates were implanted on the same location as the test group by using a micro auto-drive screw with a special screwdriver. After implantation of both test and control biomaterials into two different groups, each rat skin was then sewed by using silk 6-0 suture and antibiotic ointment was applied on top of the incision wound.
An intramuscular injection of Cephazolin (with 50 mg/Kg dosage) was applied followed by daily oral analgesics distribution using ibuprofen (15 mg/Kg dosage). Animal properties such as body weight, behavioral changes, and clinical signs, especially in the wounded area, were observed carefully by using both microscopic observation dan daily monitoring for a total of fourteen days.
Histological analysis
On the fifteenth day, all animals from botch control and test groups were euthanized intraperitoneally by using Ketamine hydrochloride (60-75 mg/Kg dosage) and 2x2 cm of calvaria bone were taken with the implant in the middle of the specimens. All specimens were then preserved in a 10% formaldehyde for further observation and analysis. These histological samples were then stained with Hematoxylin-eosin (HE) staining reagents and observed under a light- microscope apparatus. [19, 20]
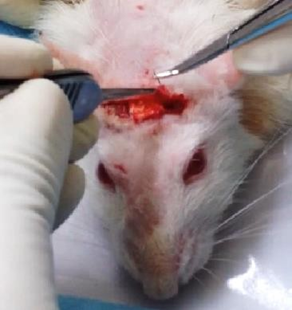
Figure 2. Implantation of mud-crab shell biomaterial on a rat calvaria bone
Statistical Analysis
Observation data obtained were then analyzed using SPSS statistical software version 21.0 for windows. Independent t-tests were used to compare foreign body giant cells originated from both control and test groups. Inflammatory infiltration value from both groups was compared by using the Kolmogorov Smirnov method.
Results
A total of fourteen Wistar strain of white male rats were successfully implanted with biomaterials plates (titanium for the control group, mud-crab shells for the test group) after the artificial injury on the calvaria bone was made. Based on the observation performed within fourteen days period: no pathologic sign, no behavioral changes, and no mortality was shown by both groups. Clinical appearance on the incisional area also showed no mild inflammatory reaction (such as erythema). The macroscopic figure of histological specimens showed that there were some foreign body reactions recognized by the amount of foreign body giant cells and also inflammatory infiltration. Different amounts of foreign body cells between both groups can be observed with the p-value, which was 0.005 lesser (p<0.005) for the control group (Table 1). Table 2 shows that the inflammatory infiltration of each group is homogenic (p= 0.938).
|
Table 1. Comparison of the mean amount of foreign body giant cell between the titanium plate group and crab shell group |
|||
|
Variable |
Group |
|
p-value |
|
Foreign Body Giant Cell |
Titanium Plate (n=7) |
Crab shell (n=7) |
|
|
Mean std |
0,714 ± 0,405 |
1,857 ± 0,790 |
0,005** |
|
Median |
0,667 |
2,00 |
|
|
Range (min-max) |
0-1,33 |
0,67 – 3,00 |
|
|
Table 2. Comparison of the mean amount of inflammatory infiltrate between the titanium plate group and crab shell |
|||
|
Variable |
Group |
p-value |
|
|
Titanium plate |
Crab shell |
||
|
Inflammatory infiltrate |
|
|
|
|
No |
0 |
0 |
|
|
Plenty |
2 (28,6%) |
2 (28,6%) |
|
|
Moderate |
3 (42,9%) |
1 (14,3%) |
0,938 |
|
Few |
2 (28,6%) |
2 (28,6%) |
|
Discussion
This study aimed to learn the biomaterial property of mud-crab (Scylla serrata) shells as a potential alternative to replace the common titanium plate. The collected biomaterial of mud-crab shells was carefully prepared through an initial deproteinization before being implanted into the artificially injured Wistar white male rats’ calvaria bone. The deproteinization process was performed to remove the non-immunogenic factors that may be contained throughout the collection procedure. Based on the previous study done by Okumus, et al., evaluating the cuttlefish spine as a xenograft material, it is essential to perform the deproteinization step for biomaterials. The use of a rat animal model in this study was meant as an assessment: to check the foreign body reactions when mud-crab shells biomaterial was introduced inside the body (in vivo experiment). Several other studies about alternative biomaterial were also performed by Dupoirieux, et al. [12, 16, 17]
One of the studies by Dupoirieux was a pilot study in an attempt to search for novel biomaterial substitution to be used in maxillofacial surgery was using chicken eggshell as a biomaterial source. The result of the study found that biomaterials originated from chicken eggshells was biocompatible to be used in filling bone defects, but it cannot be used in the weight-bearing area of the facial bone. Another study conducted in 1999, also by Dupoirieux, was trying to use ostrich eggshell originated biomaterial as an on-lay graft in rabbit mandibular bone. Unfortunately, the result was more or less similar to the one using biomaterial from chicken eggshells. In addition, the use of ostrich eggshell as a biomaterial source in the rabbit animal model was not applicable in Indonesia due to the biomaterial source rarity. [13-17]
An autologous-graft implant (commonly known as an autograft) remains as the gold standard due to its compatibility in various properties such as its osteo-genicity and osteo-inductivity. Nevertheless, the challenges in autograft application were also high due to several factors: (1) morbidity rate; (2) limited area of donor; (3) pain level; and (4) surgery time often takes long times. As an alloplastic graft material, titanium plate becomes the first line of choice to be applied to clinical surgery. It is well known to have a very good biocompatibility profile such as stability, availability, rigidity level, and easiness to shape. The only disadvantage of titanium plate is its price, which is considered to be fairly expensive. [6, 7, 9, 10, 12]
Naturally, human bones developed from cellular proteins that are bounded by extracellular matrix (ECM). The hard and soft shells of mud crabs are rich in calcium contents sine it was composed of the protein matrix and some other enzymes that control the calcium carbonate crystal growth. The other two components that also play a critical role in the formation (mineralization process) of crab eggshell are collagen and chitin. In all connective tissues, collagen has mechanical functions, providing elasticity and structure for the component tissues. Collagen is also a part of the bone matrix that assists the framework of osteoblast, osteoclast, and osteocyte. Collagens were used to give the signal of extracellular matrix production. On the other side, the chitin protein matrix from mud-crab shell possess the ability to produce and secrete organic matrix to defend integument integrity. [18, 21] A study conducted by Wilson et al. has found that the crushed mud-crab shell implanted to Sprague-Dawley subcutaneous area has good biocompatibility profiles. In this study, we are able to show the interaction profile between biomaterial from mud-crab shells particle with macrophage. Macrophages from the body of the rat animal model tried to eliminate particles from mud-crab shells by using phagocytic mechanisms, the disintegration of particles inside the lysosome. The appearance of collagen fiber and mineral particle suggests that crab shell is an effective material bone growth stimulation, quite similar to natural demineralization of bone. [18]
During or after the implantation process of biomaterials, the human body will respond to it biologically through different biochemical processes. Some of the initial response that can be observed includes: (1) acute inflammatory response (polymorphonuclear cell); (2) chronic inflammatory cell (monocyte, lymphocyte, plasma cell); and (3) foreign body reaction such as the emerge of foreign body giant cells (FBGCs). FBGCs can be observed on day seven to fourteen after the implantation procedure. Foreign body reaction usually emerged at the end of an inflammatory process and it consists of proteins that are related to adsorption processes such as monocyte adhesion, macrophage adhesion, and macrophage fusion. FBGCs is the main mediator of foreign body reaction [22, 23]. FBGC is an inflammatory cell formed by macrophages and FBGCs fusion and at the end of the process; it is transformed into multiple big cell masses. The fusion between macrophages most of the time was also affected by the surface of the implanted biomaterial. The surface of implanted biomaterial usually contains proteins that are able to facilitate FBGCs adherence. Thus, macrophage and FBGCs are combined together and then this complex will modulate the connective tissue growth while also degrading other material and encapsulating fibrous. Biocompatible material provokes an acute inflammatory reaction and faster chronic inflammatory reaction, which is no more than 2 weeks [22, 23].
This study was performed in fourteen days after implantation of both standard and test biomaterials to two different animal-model (Wistar white male rat) group. On the fifteenth day, each animal’s calvaria bone was collected for histological analysis. The method used for the histological analysis of this study was the same method developed by Uraz et al. The aim of performing a histological analysis in this study was to understand the time span related to the expected response of acute and chronic inflammatory reaction. Based on the study conducted by Anderson et al., it can be understood that a biocompatible material will show rapid resolution of acute and chronic inflammatory reactions, which usually take no longer than fourteen days.[22, 23]
Calvaria bones of the rat animal model were artificially injured by using burr until the vessel was exposed. The exposure of this vessel will allow an interaction between blood and biomaterial used in the study. As it is stated in the study of Anderson et al., the host response consists of injury process, blood-material interaction, provisional matrix formation, acute inflammatory response, chronic inflammatory response, granulation tissue formation, foreign body reaction, and fibrosis capsule formation. This procedure that indicates the artificial injury is necessary to evaluate the inflammatory response and foreign body reaction against both control and test biomaterials. The reaction profile of each titanium plate (as control biomaterial) and mud-crab shell (as test biomaterial) were evaluate separately. Banica et al. stated that an orbital reconstruction performed using titanium plate tends to give better results compared to the one that using a bone graft. So far, titanium plate is the most biocompatible material to be implanted in reconstruction but it was also quite difficult to be explanted. A retrospective study done by Yi et al. found that there was no implant rejection, infections, nor shifting of the implant on 46 patients on 6 months post-operation. This study also found a similar result in which it has shown no extrusion, no hyperemic, nor edema on macroscopic observation on the fifteenth day after treatment.[24, 25]
During the observation process by using a light microscope (with ten times magnification level), the injured area on from mud-crab shells specimen showed tighter bridging while specimens of titanium plate showed wider bridging. A study done by Neuman et al. found that implant material with a higher content of calcium phosphate has better osteoconductive properties since it has the ability to support bone regeneration and also function as a scaffold that enables migration to the defected area. Materials that have interconnecting pores size of 150-450 micrometers are ideal for this condition. Chen et al. also found that the exoskeleton component of mud-crab shells has a vertical porous tubule with higher density. It derives a long ribbon-like tubule and functions as a nutritional and ion transport channel during the bone formation process. Moreover, Wilson et al. discovered that the biomaterial from mud-crab shells is the potential to stimulate bone growth (osteo-stimulation). Tighter wound gap bridging based on the use of mud-crab shell biomaterial signifies that there is more active bone regeneration process in the test group compared to the control group.[10, 18]
Voggenreiter et al. reported that there is an immune-inflammatory reaction on the bones that were being implanted with titanium plates. The immunochemical examination was taken from the bone specimen and 15 out of 22 specimens showed moderate infiltration of cytotoxic T-lymphocyte (CD8 positive cell) and B-lymphocyte (CD79α positive cell). This is a sign of a moderate inflammatory reaction after titanium plate implantation. [26] These findings might support the result of this study, in which the inflammatory infiltration level was not significant between the control and test groups.
FBGCs level in both groups has different average numbers (mean) and it may have a correlation with its properties. Harkel et al. reported that FBGCs possess the ability to dissolve minerals such as hydroxyapatite and calcium carbonate. The dissolved minerals will then be absorbed by the bone with the osteoclast process. This study result found that the average number (mean) of FBGCs counting is higher on test group compared to the control group. [27]
However, this research only analyzed FBGCs as the main parameter of foreign body reaction and inflammatory infiltration level. Thus, further histological and immunochemical examination with longer experiment time is needed for better comprehension of the phenomenon.
Conclusion
The average number (mean) of FBGCs of mud-crab shell biomaterial implantation is higher compared to the titanium plate. Further studies needed to evaluate the crab shell as a potential alternative xenograft implant material for maxillofacial trauma.
References
- Safabakhsh M, Moudi E, Khafri S, Abesi F. Radiographic Evaluation of Maxillary Sinus Surgical Risks in Sinus Lifting Surgery Candidates Using Cone Beam Computed Tomography (CBCT). Ann. Dent. Spec. 2019;7(2):1-9.
- Kalantarzadeh ZA, Dehcheshmeh NF, Haghighizadeh MH, Mohammadi P. Analyzing the Cost of Care for Burn Injuries and Its Determinants in a University Hospital for Accidents and Burns. Entomol. Appl. Sci. Lett. 2020;6(4):18-24.
- Majed V, Abdoli G, Mahdavi G, Khodaei H. Socio-Economic Factors Affecting Road Accidents. Journal of Organizational Behavior Research. 2020;5(1)32-41.
- Yamany IA. The Employment of CBCT in Assessing Bone Loss around Dental Implants in Patients Receiving Mandibular Implant Supported over dentures. Int. J. Pharm. Res. Allied Sci. 2019;8(3):9-16.
- American Academy of Ophtalmology. Orbit, Eyelids and Lacrimal System. San Fransisco: The Foundation of the American Academy of Ophtalmology; 2011-2012;8-9, 19, 98-104.
- Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. Journal of Orthopaedic Surgery and Research. 2014; 9(18): 1-27.
- Pe LC, Cristobal S, Sunico ATC, Romero HL. Ostrich eggshell as an onlay bone-graft substitute for orbital blow-out fractures. 2004;29(3):127–30.
- Lim T, Rasheed Z, Sundar G. A Safe and Accurate Method of Assessing the Size of Implants Required in Orbital Floor Reconstruction. Craniomaxillofacial Trauma Reconstr. 2012;05(2):111–4.
- Mok D, Lessard L, Cordoba C, Harris PG, Nikolis A. A review of materials currently used in orbital floor reconstruction. Can J Plast Surg. 2004;12(3):134–40.
- Neumann A. Biomaterials for craniofacial reconstruction. Laryngo-Rhino- Otologie. 2009;88:S48–63.
- Mir HS, Avashia YJ, Thaller SR, Fan KL, Sastry A. Materials Used for Reconstruction After Orbital Floor Fracture. J Craniofac Surg. 2012;23(7):S49– 55.
- Okumuş Z, Yildirim ÖmS. The cuttlefish backbone: A new bone xenograft material? Turkish J Vet Anim Sci. 2005;29(5):1177–84.
- Dupoirieux L. Powdered eggshell. A pilot study on a new bone substitute for use in maxillofacial surgery. Journal of Cranio-Maxillofacial Surgery. 1995; 23(3): 187-194.
- Dupoirieux L. Ostrich eggshell as a bone substitute: A preliminary report of its biological behavior in animals - a possibility in facial reconstructive surgery, British Jounal of Oral & Maxillofacial Surgery. 1999; 37(6): 467-471.
- Nakano T, Ikawa NI, Ozimek L. Chemical composition of chicken eggshell and shell membranes. Poult Sci. 2003;82(3):510–4.
- Park JW, Bae SR, Suh JY, Lee DH, Kim SH, Kim H, Lee CS. Evaluation of bone healing with eggshell-derived bone graft substitutes in rat calvaria: A pilot study. Journal of Biomedical Materials Research Part A. 2008 Oct;87(1):203-14..
- Uraz A, Gultekin SE, Senguven B, Karaduman B, Sofuoglu IP, Pehlivan S, et al. Histologic and histomorphometric assessment of eggshell-derived bone graft substitutes on bone healing in rats. J Clin Exp Dent. 2013 Feb;5(1):e23..
- Wilson AC, Guysa A, Mehl P, Anderson W. An initial assessment of the biocompatibility of crab shell for bone tissue engineering. Material Science and Engineering. 2011;32(2):78-82
- Atik N, Maqrizi DS. Effect of guava extract administration on megakaryocytes amount in mice femur. IJCP. 2017 Jun 1;6(2):116-22.
- Avriyanti E, Atik N, Kunii M, Furumoto N, Iwano T, Yoshimura SI, Harada R, Harada A. Functional redundancy of protein kinase D1 and protein kinase D2 in neuronal polarity. Neuroscience research. 2015 Jun 1;95:12-20
- Ge H, Zhao B, Lai Y, Hu X, Zhang D, Hu K. From crabshell to chitosan- hydroxyapatite composite material via a biomorphic mineralization synthesis method. Journal of Material Science and Engineering: Mater Med. 2010;21(6) 1781-1787.
- Anderson JM. Foreign body reaction to biometarials. Semin Immunol. 2008;20(2):86–100.
- Hu WJ, Eaton JW, Tang L. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001;98(4):1231–8.
- Banica B, Ene P, Vranceanu D, Ene R. Titanium preformed implants in orbital floor reconstruction - case presentation, review of literature. Maedica (Buchar) [Internet]. 2013;8(1):34–9.
- Yi WS, Xu XL, Ma JR, Ou XR. Reconstruction of complex orbital fracture with titanium implants. Int J Ophthalmol [Internet]. 2012;5(4):488–92..
- Voggenreiter G, Leiting S, Brauer H, Leiting P, Majetschak M. Immuno- inflammatory tissue reaction to stainless-steel and titanium plates used for internal fixation of long bones. Biomaterials. 2003;24(2):247-254.
- Harkel B, Schoenmaker T, Picavet NL, Davidson NL, Vries TJ, Everts V. The Foreign Nody Giant Cell Cannot Resorb Bone, Bur Dissolves Hydroxyapatite Like Osteoclast. Journal Pone. 2015;10(10): e0139564.
Figure 1. An image of mud-crab shells (in a plate form) final product
The titanium plates used in this study were the Osteomed Craniomaxillofacial CFXTM. The microplates have 18 holes with 1,2 mm diameter and were cut down into a 10x4 mm size per plate by using plate scissors.
Artificial calvarian bone injury
Male Wistar rats were anesthetized with an intraperitoneal dose of Ketamine hydrochloride (0,3 ml/100 gram of body weight) and Xylazine hydrochloride 2% (5-10 mg/Kg of body weight). Rats were then placed under a microscope for minor surgery and were given povidone-iodine (as an antiseptic) on the calvaria bone area. Two centimeters of linear-horizontal incision was made at the frontal area subcutis of each rat. Then, an incision at the periosteum part was made and continued with a dissection by using a dissector on the top part of calvaria bone. The artificial injury size of each rat was 10 mm and it was made by using a drill. The injury could be observed from the bleeding of intraosseous blood vessels.
For the test group, the mud-crab shell biomaterials were implanted under the periosteum, above the injured bone. While for the control group, titanium plates were implanted on the same location as the test group by using a micro auto-drive screw with a special screwdriver. After implantation of both test and control biomaterials into two different groups, each rat skin was then sewed by using silk 6-0 suture and antibiotic ointment was applied on top of the incision wound.
An intramuscular injection of Cephazolin (with 50 mg/Kg dosage) was applied followed by daily oral analgesics distribution using ibuprofen (15 mg/Kg dosage). Animal properties such as body weight, behavioral changes, and clinical signs, especially in the wounded area, were observed carefully by using both microscopic observation dan daily monitoring for a total of fourteen days.
Histological analysis
On the fifteenth day, all animals from botch control and test groups were euthanized intraperitoneally by using Ketamine hydrochloride (60-75 mg/Kg dosage) and 2x2 cm of calvaria bone were taken with the implant in the middle of the specimens. All specimens were then preserved in a 10% formaldehyde for further observation and analysis. These histological samples were then stained with Hematoxylin-eosin (HE) staining reagents and observed under a light- microscope apparatus. [19, 20]
Figure 2. Implantation of mud-crab shell biomaterial on a rat calvaria bone
Statistical Analysis
Observation data obtained were then analyzed using SPSS statistical software version 21.0 for windows. Independent t-tests were used to compare foreign body giant cells originated from both control and test groups. Inflammatory infiltration value from both groups was compared by using the Kolmogorov Smirnov method.
Results
A total of fourteen Wistar strain of white male rats were successfully implanted with biomaterials plates (titanium for the control group, mud-crab shells for the test group) after the artificial injury on the calvaria bone was made. Based on the observation performed within fourteen days period: no pathologic sign, no behavioral changes, and no mortality was shown by both groups. Clinical appearance on the incisional area also showed no mild inflammatory reaction (such as erythema). The macroscopic figure of histological specimens showed that there were some foreign body reactions recognized by the amount of foreign body giant cells and also inflammatory infiltration. Different amounts of foreign body cells between both groups can be observed with the p-value, which was 0.005 lesser (p<0.005) for the control group (Table 1). Table 2 shows that the inflammatory infiltration of each group is homogenic (p= 0.938).
|
Table 1. Comparison of the mean amount of foreign body giant cell between the titanium plate group and crab shell group |
|||
|
Variable |
Group |
|
p-value |
|
Foreign Body Giant Cell |
Titanium Plate (n=7) |
Crab shell (n=7) |
|
|
Mean std |
0,714 ± 0,405 |
1,857 ± 0,790 |
0,005** |
|
Median |
0,667 |
2,00 |
|
|
Range (min-max) |
0-1,33 |
0,67 – 3,00 |
|
|
Table 2. Comparison of the mean amount of inflammatory infiltrate between the titanium plate group and crab shell |
|||
|
Variable |
Group |
p-value |
|
|
Titanium plate |
Crab shell |
||
|
Inflammatory infiltrate |
|
|
|
|
No |
0 |
0 |
|
|
Plenty |
2 (28,6%) |
2 (28,6%) |
|
|
Moderate |
3 (42,9%) |
1 (14,3%) |
0,938 |
|
Few |
2 (28,6%) |
2 (28,6%) |
|
Discussion
This study aimed to learn the biomaterial property of mud-crab (Scylla serrata) shells as a potential alternative to replace the common titanium plate. The collected biomaterial of mud-crab shells was carefully prepared through an initial deproteinization before being implanted into the artificially injured Wistar white male rats’ calvaria bone. The deproteinization process was performed to remove the non-immunogenic factors that may be contained throughout the collection procedure. Based on the previous study done by Okumus, et al., evaluating the cuttlefish spine as a xenograft material, it is essential to perform the deproteinization step for biomaterials. The use of a rat animal model in this study was meant as an assessment: to check the foreign body reactions when mud-crab shells biomaterial was introduced inside the body (in vivo experiment). Several other studies about alternative biomaterial were also performed by Dupoirieux, et al. [12, 16, 17]
One of the studies by Dupoirieux was a pilot study in an attempt to search for novel biomaterial substitution to be used in maxillofacial surgery was using chicken eggshell as a biomaterial source. The result of the study found that biomaterials originated from chicken eggshells was biocompatible to be used in filling bone defects, but it cannot be used in the weight-bearing area of the facial bone. Another study conducted in 1999, also by Dupoirieux, was trying to use ostrich eggshell originated biomaterial as an on-lay graft in rabbit mandibular bone. Unfortunately, the result was more or less similar to the one using biomaterial from chicken eggshells. In addition, the use of ostrich eggshell as a biomaterial source in the rabbit animal model was not applicable in Indonesia due to the biomaterial source rarity. [13-17]
An autologous-graft implant (commonly known as an autograft) remains as the gold standard due to its compatibility in various properties such as its osteo-genicity and osteo-inductivity. Nevertheless, the challenges in autograft application were also high due to several factors: (1) morbidity rate; (2) limited area of donor; (3) pain level; and (4) surgery time often takes long times. As an alloplastic graft material, titanium plate becomes the first line of choice to be applied to clinical surgery. It is well known to have a very good biocompatibility profile such as stability, availability, rigidity level, and easiness to shape. The only disadvantage of titanium plate is its price, which is considered to be fairly expensive. [6, 7, 9, 10, 12]
Naturally, human bones developed from cellular proteins that are bounded by extracellular matrix (ECM). The hard and soft shells of mud crabs are rich in calcium contents sine it was composed of the protein matrix and some other enzymes that control the calcium carbonate crystal growth. The other two components that also play a critical role in the formation (mineralization process) of crab eggshell are collagen and chitin. In all connective tissues, collagen has mechanical functions, providing elasticity and structure for the component tissues. Collagen is also a part of the bone matrix that assists the framework of osteoblast, osteoclast, and osteocyte. Collagens were used to give the signal of extracellular matrix production. On the other side, the chitin protein matrix from mud-crab shell possess the ability to produce and secrete organic matrix to defend integument integrity. [18, 21] A study conducted by Wilson et al. has found that the crushed mud-crab shell implanted to Sprague-Dawley subcutaneous area has good biocompatibility profiles. In this study, we are able to show the interaction profile between biomaterial from mud-crab shells particle with macrophage. Macrophages from the body of the rat animal model tried to eliminate particles from mud-crab shells by using phagocytic mechanisms, the disintegration of particles inside the lysosome. The appearance of collagen fiber and mineral particle suggests that crab shell is an effective material bone growth stimulation, quite similar to natural demineralization of bone. [18]
During or after the implantation process of biomaterials, the human body will respond to it biologically through different biochemical processes. Some of the initial response that can be observed includes: (1) acute inflammatory response (polymorphonuclear cell); (2) chronic inflammatory cell (monocyte, lymphocyte, plasma cell); and (3) foreign body reaction such as the emerge of foreign body giant cells (FBGCs). FBGCs can be observed on day seven to fourteen after the implantation procedure. Foreign body reaction usually emerged at the end of an inflammatory process and it consists of proteins that are related to adsorption processes such as monocyte adhesion, macrophage adhesion, and macrophage fusion. FBGCs is the main mediator of foreign body reaction [22, 23]. FBGC is an inflammatory cell formed by macrophages and FBGCs fusion and at the end of the process; it is transformed into multiple big cell masses. The fusion between macrophages most of the time was also affected by the surface of the implanted biomaterial. The surface of implanted biomaterial usually contains proteins that are able to facilitate FBGCs adherence. Thus, macrophage and FBGCs are combined together and then this complex will modulate the connective tissue growth while also degrading other material and encapsulating fibrous. Biocompatible material provokes an acute inflammatory reaction and faster chronic inflammatory reaction, which is no more than 2 weeks [22, 23].
This study was performed in fourteen days after implantation of both standard and test biomaterials to two different animal-model (Wistar white male rat) group. On the fifteenth day, each animal’s calvaria bone was collected for histological analysis. The method used for the histological analysis of this study was the same method developed by Uraz et al. The aim of performing a histological analysis in this study was to understand the time span related to the expected response of acute and chronic inflammatory reaction. Based on the study conducted by Anderson et al., it can be understood that a biocompatible material will show rapid resolution of acute and chronic inflammatory reactions, which usually take no longer than fourteen days.[22, 23]
Calvaria bones of the rat animal model were artificially injured by using burr until the vessel was exposed. The exposure of this vessel will allow an interaction between blood and biomaterial used in the study. As it is stated in the study of Anderson et al., the host response consists of injury process, blood-material interaction, provisional matrix formation, acute inflammatory response, chronic inflammatory response, granulation tissue formation, foreign body reaction, and fibrosis capsule formation. This procedure that indicates the artificial injury is necessary to evaluate the inflammatory response and foreign body reaction against both control and test biomaterials. The reaction profile of each titanium plate (as control biomaterial) and mud-crab shell (as test biomaterial) were evaluate separately. Banica et al. stated that an orbital reconstruction performed using titanium plate tends to give better results compared to the one that using a bone graft. So far, titanium plate is the most biocompatible material to be implanted in reconstruction but it was also quite difficult to be explanted. A retrospective study done by Yi et al. found that there was no implant rejection, infections, nor shifting of the implant on 46 patients on 6 months post-operation. This study also found a similar result in which it has shown no extrusion, no hyperemic, nor edema on macroscopic observation on the fifteenth day after treatment.[24, 25]
During the observation process by using a light microscope (with ten times magnification level), the injured area on from mud-crab shells specimen showed tighter bridging while specimens of titanium plate showed wider bridging. A study done by Neuman et al. found that implant material with a higher content of calcium phosphate has better osteoconductive properties since it has the ability to support bone regeneration and also function as a scaffold that enables migration to the defected area. Materials that have interconnecting pores size of 150-450 micrometers are ideal for this condition. Chen et al. also found that the exoskeleton component of mud-crab shells has a vertical porous tubule with higher density. It derives a long ribbon-like tubule and functions as a nutritional and ion transport channel during the bone formation process. Moreover, Wilson et al. discovered that the biomaterial from mud-crab shells is the potential to stimulate bone growth (osteo-stimulation). Tighter wound gap bridging based on the use of mud-crab shell biomaterial signifies that there is more active bone regeneration process in the test group compared to the control group.[10, 18]
Voggenreiter et al. reported that there is an immune-inflammatory reaction on the bones that were being implanted with titanium plates. The immunochemical examination was taken from the bone specimen and 15 out of 22 specimens showed moderate infiltration of cytotoxic T-lymphocyte (CD8 positive cell) and B-lymphocyte (CD79α positive cell). This is a sign of a moderate inflammatory reaction after titanium plate implantation. [26] These findings might support the result of this study, in which the inflammatory infiltration level was not significant between the control and test groups.
FBGCs level in both groups has different average numbers (mean) and it may have a correlation with its properties. Harkel et al. reported that FBGCs possess the ability to dissolve minerals such as hydroxyapatite and calcium carbonate. The dissolved minerals will then be absorbed by the bone with the osteoclast process. This study result found that the average number (mean) of FBGCs counting is higher on test group compared to the control group. [27]
However, this research only analyzed FBGCs as the main parameter of foreign body reaction and inflammatory infiltration level. Thus, further histological and immunochemical examination with longer experiment time is needed for better comprehension of the phenomenon.
Conclusion
The average number (mean) of FBGCs of mud-crab shell biomaterial implantation is higher compared to the titanium plate. Further studies needed to evaluate the crab shell as a potential alternative xenograft implant material for maxillofacial trauma.
References
- Safabakhsh M, Moudi E, Khafri S, Abesi F. Radiographic Evaluation of Maxillary Sinus Surgical Risks in Sinus Lifting Surgery Candidates Using Cone Beam Computed Tomography (CBCT). Ann. Dent. Spec. 2019;7(2):1-9.
- Kalantarzadeh ZA, Dehcheshmeh NF, Haghighizadeh MH, Mohammadi P. Analyzing the Cost of Care for Burn Injuries and Its Determinants in a University Hospital for Accidents and Burns. Entomol. Appl. Sci. Lett. 2020;6(4):18-24.
- Majed V, Abdoli G, Mahdavi G, Khodaei H. Socio-Economic Factors Affecting Road Accidents. Journal of Organizational Behavior Research. 2020;5(1)32-41.
- Yamany IA. The Employment of CBCT in Assessing Bone Loss around Dental Implants in Patients Receiving Mandibular Implant Supported over dentures. Int. J. Pharm. Res. Allied Sci. 2019;8(3):9-16.
- American Academy of Ophtalmology. Orbit, Eyelids and Lacrimal System. San Fransisco: The Foundation of the American Academy of Ophtalmology; 2011-2012;8-9, 19, 98-104.
- Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. Journal of Orthopaedic Surgery and Research. 2014; 9(18): 1-27.
- Pe LC, Cristobal S, Sunico ATC, Romero HL. Ostrich eggshell as an onlay bone-graft substitute for orbital blow-out fractures. 2004;29(3):127–30.
- Lim T, Rasheed Z, Sundar G. A Safe and Accurate Method of Assessing the Size of Implants Required in Orbital Floor Reconstruction. Craniomaxillofacial Trauma Reconstr. 2012;05(2):111–4.
- Mok D, Lessard L, Cordoba C, Harris PG, Nikolis A. A review of materials currently used in orbital floor reconstruction. Can J Plast Surg. 2004;12(3):134–40.
- Neumann A. Biomaterials for craniofacial reconstruction. Laryngo-Rhino- Otologie. 2009;88:S48–63.
- Mir HS, Avashia YJ, Thaller SR, Fan KL, Sastry A. Materials Used for Reconstruction After Orbital Floor Fracture. J Craniofac Surg. 2012;23(7):S49– 55.
- Okumuş Z, Yildirim ÖmS. The cuttlefish backbone: A new bone xenograft material? Turkish J Vet Anim Sci. 2005;29(5):1177–84.
- Dupoirieux L. Powdered eggshell. A pilot study on a new bone substitute for use in maxillofacial surgery. Journal of Cranio-Maxillofacial Surgery. 1995; 23(3): 187-194.
- Dupoirieux L. Ostrich eggshell as a bone substitute: A preliminary report of its biological behavior in animals - a possibility in facial reconstructive surgery, British Jounal of Oral & Maxillofacial Surgery. 1999; 37(6): 467-471.
- Nakano T, Ikawa NI, Ozimek L. Chemical composition of chicken eggshell and shell membranes. Poult Sci. 2003;82(3):510–4.
- Park JW, Bae SR, Suh JY, Lee DH, Kim SH, Kim H, Lee CS. Evaluation of bone healing with eggshell-derived bone graft substitutes in rat calvaria: A pilot study. Journal of Biomedical Materials Research Part A. 2008 Oct;87(1):203-14..
- Uraz A, Gultekin SE, Senguven B, Karaduman B, Sofuoglu IP, Pehlivan S, et al. Histologic and histomorphometric assessment of eggshell-derived bone graft substitutes on bone healing in rats. J Clin Exp Dent. 2013 Feb;5(1):e23..
- Wilson AC, Guysa A, Mehl P, Anderson W. An initial assessment of the biocompatibility of crab shell for bone tissue engineering. Material Science and Engineering. 2011;32(2):78-82
- Atik N, Maqrizi DS. Effect of guava extract administration on megakaryocytes amount in mice femur. IJCP. 2017 Jun 1;6(2):116-22.
- Avriyanti E, Atik N, Kunii M, Furumoto N, Iwano T, Yoshimura SI, Harada R, Harada A. Functional redundancy of protein kinase D1 and protein kinase D2 in neuronal polarity. Neuroscience research. 2015 Jun 1;95:12-20
- Ge H, Zhao B, Lai Y, Hu X, Zhang D, Hu K. From crabshell to chitosan- hydroxyapatite composite material via a biomorphic mineralization synthesis method. Journal of Material Science and Engineering: Mater Med. 2010;21(6) 1781-1787.
- Anderson JM. Foreign body reaction to biometarials. Semin Immunol. 2008;20(2):86–100.
- Hu WJ, Eaton JW, Tang L. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001;98(4):1231–8.
- Banica B, Ene P, Vranceanu D, Ene R. Titanium preformed implants in orbital floor reconstruction - case presentation, review of literature. Maedica (Buchar) [Internet]. 2013;8(1):34–9.
- Yi WS, Xu XL, Ma JR, Ou XR. Reconstruction of complex orbital fracture with titanium implants. Int J Ophthalmol [Internet]. 2012;5(4):488–92..
- Voggenreiter G, Leiting S, Brauer H, Leiting P, Majetschak M. Immuno- inflammatory tissue reaction to stainless-steel and titanium plates used for internal fixation of long bones. Biomaterials. 2003;24(2):247-254.
- Harkel B, Schoenmaker T, Picavet NL, Davidson NL, Vries TJ, Everts V. The Foreign Nody Giant Cell Cannot Resorb Bone, Bur Dissolves Hydroxyapatite Like Osteoclast. Journal Pone. 2015;10(10): e0139564.
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]