|
Original Article |
Acapella versus hand-held positive expiratory pressure on Pulmonary functions in management of chronic obstructive Pulmonary diseases
Mohamed Shamakh1*, Nagwa Badr2, Mohamed El-Batanouny3, Mohamed Shendy4
1 Assistant Lecturer of Physical Therapy for Cardio-respiratory Disorders and Geriatrics, Faculty of Physical Therapy, Cairo University, Cairo, Egypt. 2Professor of Physical Therapy for Cardio-respiratory Disorders and Geriatrics, Faculty of Physical Therapy, Cairo University, Cairo, Egypt. 3Professor of Chest Diseases, Faculty of Medicine, Kasr Al Aini, Cairo University, Cairo, Egypt. 4Assistant Professor of Physical Therapy for Cardio-respiratory Disorders and Geriatrics, Cairo University, Egypt (Permanent) and Faculty of Medical Rehabilitation Sciences, Taibah University, KSA(Stationary).
Correspondence: Mohamed Shamakh, Department of Physical Therapy for Cardio-respiratory Disorders and Geriatrics, Faculty of Physical Therapy, Cairo University, Cairo, Egypt. Email: Mohamed.shamakh @ cu.edu.eg.
|
ABSTRACT
Background: Chronic Obstructive Pulmonary Disease (COPD) is a progressive disease that needs a multidisciplinary approach with an increasing emphasis on self-management. Physiotherapists utilize numerous airway clearance techniques in COPD management like Acapella and positive expiratory pressure (PEP). Objective: To find any difference between Acapella and hand-held positive expiratory pressure in the management of moderate COPD. Study design: a single-blinded randomized controlled trial. Methods: 60 patients were recruited from Cairo University Hospitals, Egypt, from August 2016 to February 2019. They were randomly classified into three equal groups. Group A patients received routine treatment: Active cycle of breathing technique (ACBT). Groups B and C received ACBT and either PEP or Acapella, respectively. Outcome measures were measured after an acute exacerbation and after six months of self-management at home. Results: FEV1 improved in all groups; A: 2.64%, B: 8.92%, and C: 10.49%. There was no significant difference between groups B and C (P=0.619). FEV1/FVC improved in all groups; A: 3.71%, B: 7.73%, and C: 8.52%. There was no significant difference between groups B and C (P=0.286). Walking distance in the six-minute walk test increased in all groups; A: 3%, B: 12.95%, and C: 20.09%. There was no significant difference between groups B and C (P=0.659). Quality of life using COPD assessment test scores revealed improvements in the three groups. All groups’ test scores decreased; A: 4.36%, B: 22.85%, and C: 22.99%. There was no significant difference between groups B and C (P=0.010). Conclusion: PEP and Acapella improved moderate COPD pulmonary functions. ACBT alone showed improvements but to a lesser extent than Acapella and PEP.
Keywords: PEP, Threshold PEP, COPD, Active cycle breathing technique, ACBT |
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a non-communicable progressive [1, 2] and non-curable disease,[3] which is characterized by persistent respiratory symptoms and airflow limitation.[4] It is potentially fatal, and currently, it comes after stroke, ischemic heart diseases, and lower respiratory infections as the fourth cause of death in the world. It claimed three million lives in 2016.[1, 3, 5] Various investigations have verified that poor oral hygiene,[6] as well as carotid artery intima-media thickness,[7] are associated with an increased risk of COPD. Besides, Chlamydophila pneumoniae is one of the gram-negative bacteria that can result in COPD.[8]
COPD management requires a multidisciplinary approach [9] with an increasing emphasis on self-management due to increased burden on hospitals.[10] Physiotherapeutic management of COPD may comprise of sputum clearance techniques where copious secretions lead to distress.[9] Airway clearance techniques are intended to decrease the complications of secretions retention, which can include airflow obstruction, wheeze, respiratory infection, dyspnea, fatigue, and reduced quality of life.[11] Clearance methods include modalities such as conventional chest physical therapy (CPT), active cycle of breathing technique (ACBT), positive expiratory pressure (PEP), and intrapulmonary percussive ventilation. Techniques may be classified in several ways and are often utilized in concert in individuals with COPD.[12] 77% of the UK physiotherapists routinely apply these techniques in COPD treatment.[13] To date, there are no clinical trials comparing Acapella and PEP in COPD. Research on Medline database and EBSCO database "business source complete" did not show any study that compared the two techniques in the management of COPD.
This study aimed to give a better understanding of the impact of both Acapella and hand-held PEP. It compared them with respect to the patient's pulmonary functions, exercise tolerance, and impact on the quality of life. Therefore, the aim of this investigation was to answer the research question: Is there any difference between Acapella and hand-held PEP in the management of chronic obstructive pulmonary diseases?
Materials and Methods:
Sixty patients who were diagnosed with COPD in Cairo University Hospital, Kasr El Einy Hospital, Cairo, Egypt, through a period of 30 months (from August 2016 to February 2019) were included in the investigation. They were assigned to three groups; Group A receiving ACBT only, Group B receiving ACBT and PEP, and Group C receiving ACBT and Acapella.
- Subjects:
Sixty COPD, who were diagnosed with acute exacerbation of COPD and met the following criteria, were included in the research:
Inclusion criteria:
- Smokers with a history of at least 10 packs per year.
- Moderate COPD patients with COPD Stage II: FEV1/FVC < 70% of predicted & FEV1 < 80% of predicted.
- The age range of 40-65.
- Body Mass index > 18 kg/m2 and < 29 kg/m2.
Exclusion criteria:
- Mild or severe COPD.
- Patients who required invasive or non-invasive ventilation.
- Non-symptomatic COPD.
- Heart Failure Patients.
- Body Mass index > 24.9 kg/m2 and < 18 kg/m2.
- Contraindication to positive pressure.
- Instrumentation:
- Treatment equipment: Acapella (DHD Healthcare).
- Threshold PEP (Respironics).
- Vitalograph ALPHA Model 6000.
- Questionnaire: COPD Assessment Tool.
Procedures:
Moderate COPD patients with acute exacerbation were screened for eligibility. Then Microsoft Excel was used to generate a unique number for each patient. The researcher then used the number to pull pre-allocated concealed envelopes. The double-sealed envelope containing one piece of paper indicated allocation into one of the three study groups: PEEP, Acapella, and control. Treatment was done according to the patient’s needs. For follow up, outpatient visits were offered to all patients. In the case of admission to hospital with an exacerbation, their therapy would continue according to their initial allocation. Patients were given their exercise sheets and equipment and they were followed up six months after inclusion.
ACBT was developed in 1992, to combine forced exhalations with breathing exercises.[14] ACBT was utilized always or often by 88% in the UK for admission with acute exacerbation of COPD.[15] It has tidal breathing, thoracic expansions, and forced expiration techniques (Figure 1).[16] The literature shows it improves lung function, arterial blood gases, exercise tolerance, and dyspnoea scores in COPD patients.[17]
Figure 1: The active cycle of breathing.[18]
Expiratory positive pressure is a positive pressure ventilatory support that may decrease inspiratory workload.[19] It has been indicated to aid sputum clearance.[17] Threshold PEP utilizes a one-way valve with a dial that can be applied to adjust pressure settings from 5 up to 41 cm H₂O. The pressure was increased within the patient comfort limits. During treatment, the patient inhaled a full breath, then exhaled through Threshold PEP for 10-12 breaths. The patient then did 2-3 forced expiration at the end of the session. Forced expiration is thought to prevent airway collapse, to redirect air to atelectatic lung tissue, and to encourage homogenous ventilation.[20, 21]
The green Acapella (Figure 2) was used in the study as it was designed for patients with moderate COPD, who can maintain the expiratory flow of at least 15 L/min for 3 seconds. Acapella oscillations provide expiratory vibration, aiding sputum movement into larger airways.[17, 22]
The vibrations enhance more with higher flows as compared to increasing the Acapella dial settings.[23] It can be utilized in any position, as is not gravity dependent.[24, 25]
The patients performed relaxed diaphragmatic breathing at which he/she inhaled a deep breath, but less than total lung capacity. The patient then held the breath for 2-3 seconds followed by exhalation to functional residual capacity actively, but not too forcefully, through the device. The patient did 2-3 huffs at the end of the session.
Statistical analysis was done using SPSS with the p-value set at < 0.05. Ethical approval was obtained by the Research Ethics Committee of the Faculty of Physical Therapy, Cairo University, Egypt.
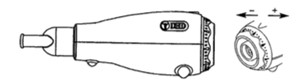
Figure 2: Acapella and dial to change positive expiratory pressure.[24]
Results:
Participants:
Sixty patients were included with baseline characteristics as in Table 1. Patients’ age ranged from 40 to 65 years. COPD prevalence increased with age, around 6.6% in 45–54 years old.[26] COPD-related symptoms prevalence was 15.7% in Egyptians over 40 years old.[27] Patients with high BMI were excluded from this investigation because it was related to a decline in lung function.[28] Obesity is thought to change the respiratory mechanics which is manifested with a reduction in respiratory muscle strength, endurance, and limitations in pulmonary function tests and exercise capacity.[29] Besides, underweight patients were excluded. COPD patients lose weight mostly because of skeletal muscle atrophy, increased inflammatory cells, modified regenerative capacity, and protein metabolism imbalance.[30] Underweight patients suffer more frequent acute exacerbations, which leads to a further decline in FEV1, impaired quality of life,[31] and high mortality.[31, 32]
Results:
All the patients were evaluated six weeks after an acute exacerbation and after six months of home self-management utilizing a spirometer, six-minute walk test (6MWT), and COPD assessment test (CAT) (Table 2). In-between groups’ mean difference and any significant levels of gratitude in Table 3. Pulmonary function tests: FEV1 improved in all groups; A: 2.64%, B: 8.92%, and C: 10.49%. There was no significant difference between groups B and C (P=0.619). Groups B and C had a significant difference from Group A (P=0.001 and P=0.008). FEV1/FVC improved in all groups; A: 3.71%, B: 7.73%, and C: 8.52%. There was no significant difference between groups B and C (P=0.286). Groups B and C had a significant difference from group A (P=0.024 and P=0.001). Walking distance in 6MWT improved in all groups; A: 3%, B: 12.95%, and C: 20.09%. There was no significant difference between groups B and C (P=0.659). Groups B and C had a significant difference from group A (P=0.004 and P=0.013). Quality of life using COPD assessment test scores showed improvements in all groups. All groups' test scores decreased; A: 4.36%, B: 22.85%, and C: 22.99%. There was no significant difference between groups B and C (P=0.010). Groups B and C had a significant difference from group A (P=0.001 and P=0.001).
|
Table 1: The baseline characteristics of the groups. P-value was tested at confidence interval=95% (CI=95%). BMI: body mass index. |
||||
|
Variable |
A |
B |
C |
P-value |
|
Age (yr) |
52.20 ± 6.81 |
53.65± 5.67 |
51.25± 5.11 |
0.439 |
|
BMI (kg/m2) |
23.08 ± 1.67 |
22.50 ± 1.48 |
22.30 ± 1.95 |
0.335 |
|
FEV1 (%) |
65.89±4.73 |
66.27±4.81 |
64.68±4.30 |
0.529 |
|
FEV1/FVC (%) |
58.22± 4.99 |
59.52± 5.49 |
60.68± 4.88 |
0.323 |
|
6MWT (m) |
302.80± 40.07 |
314.70± 49.93 |
290.65± 36.80 |
0. 213 |
|
CAT |
18.35± 2.94 |
17.95± 2.78 |
19.35± 3.57 |
0. 350 |
|
Table 2: Pre- and post-study mean ±SD values of all variables in all groups. |
|||||||||
|
Variable |
A |
B |
C |
||||||
|
Pre |
Post |
P-value |
Pre |
Post |
P-value |
Pre |
Post |
P-value |
|
|
FEV1 (%) |
65.89 ±4.73 |
67.63 ±4.84 |
0.035 * |
66.27 ±4.81 |
72.18 ±3.96 |
<0.001* |
64.68 ±4.30 |
71.47 ±4.45 |
<0.001* |
|
FEV1/FVC (%) |
58.22 ±4.9 |
60.38 ±5.01 |
0.001* |
59.52 ±5.49 |
64.12 ±5.06 |
<0.001* |
60.68 ±4.88 |
65.85 ±5.18 |
<0.001* |
|
6MWT (m) |
302.80 ±40 |
311.90 ±39.4 |
0.028* |
314.70 ±49.9 |
355.45 ±45.3 |
<0.001* |
290.65 ±36.8 |
349.05 ±51.2 |
<0.001* |
|
CAT |
18.35 ±2.94 |
17.55 ±3.5 |
0.039* |
17.95 ±2.78 |
13.85 ±2.45 |
<0.001* |
19.35 ±3.57 |
14.90 ±3.33 |
<0.001* |
Data are expressed in means ±SD, FEV1/FVC: Forced expiratory volume in the first second/forced vital capacity, 6MWT: Six-minute walk test, CAT: COPD assessment test, *Significant: p ≤0.05.
|
Table 3: Multiple-comparison post-hoc tests. |
||||
|
Variable |
Group |
Mean Difference |
Sig. |
|
|
FEV1 |
A |
B |
-4.5430* |
.002 |
|
A |
C |
-3.8415* |
.008 |
|
|
C |
A |
3.8415* |
.008 |
|
|
C |
B |
-.7015 |
.619 |
|
|
FEV1FVC |
A |
B |
-3.73550* |
.024 |
|
A |
C |
-5.46800* |
.001 |
|
|
C |
A |
5.46800* |
.001 |
|
|
C |
B |
1.73250 |
.286 |
|
|
MWT |
A |
B |
-43.550* |
.004 |
|
A |
C |
-37.150* |
.013 |
|
|
C |
A |
37.150* |
.013 |
|
|
C |
B |
-6.400 |
.659 |
|
|
CAT |
A |
B |
3.70000* |
.000 |
|
A |
C |
2.65000* |
.010 |
|
|
C |
A |
-2.65000* |
.010 |
|
|
C |
B |
1.05000 |
.294 |
|
*. The mean difference is significant at the 0.05 level.
Discussion:
Pulmonary Function:
Pulmonary function testing outcomes including FEV1 and FEV1/FVC ratio showed a significant increase in all groups with no significant difference between the two treatment groups (B and C), while there were significant differences between the control group A and either of B and C.
Regarding ACBT and improvement in pulmonary functions, several investigations were in agreement with the present research.[33-35] These may be attributed to reducing airway resistance by loosening secretions.[33, 35, 36] ACBT moves the secretions to the upper airways to be coughed out by allowing air to reach the small airways such as bronchiolar of Lambart and pores of Kohn.[36] It also enhances the strength of respiratory muscles and reduces the air trapping.[37] This results in the improvement of lung compliance and reduction of airway resistance.[37, 38]
Threshold PEP previously showed an increased FEV1 with fewer COPD exacerbations when compared to diaphragmatic breathing.[39] However, PEP didn’t show a similar result when used for a short duration.[40] These improvements may be attributed to the physiological or mechanical influences of positive pressure. PEP improved emptying of the lung, decreased functional residual capacity, enhanced tidal volume, and enabled better alveolar emptying.[41, 42] Another possible mechanism was building up of equilibrium between outward recoil of the chest wall and inward recoil of the lungs.[41] This gives the already flattened diaphragm a mechanical advantage and hence helped maximal inspiratory force.[42] It has been suggested that PEP therapy produces a positive back pressure during expiration that splints open the peripheral airways, temporarily enhancing lung volume and allowing air to move behind the obstructed lung segments through collateral ventilation pathways.[43, 44]
Acapella also improved the pulmonary function tests similar to a previous study which compared Acapella and usual airway clearance during acute exacerbation in COPD.[45] Also, Acapella demonstrated better improvements in FEV1 and more reduction of symptoms in COPD patients versus diaphragmatic breathing exercises.[46] This might be explained by the vibrations of Acapella which shakes mucus plugs and makes the patient coughs.[47, 48] The oscillations to exhaled air interrupt the airflow accelerations, acting as a series of mini-coughs in the distal airways, rather than conventional coughs working on the larger airways.[49] Therefore, this improves cough effectiveness and pushes the secretion out.[47] Acapella can be more effective for secretion mobilization because it elicits vibrations of amplitude from 5 to 8 cm H2O.[50] These vibrations increase secretion removal by reducing the viscosity of secretions and help to displace them into the airway lumen.[48]
Functional Capacity:
Walking distance in 6MWT revealed significant improvements in both the treatment groups B and C and the control group A. There was no significant difference between the two treatment groups, as well as a significant difference between the two treatment groups and the control group. This was comparable with the previous investigations which indicated that ACBT has significant and remarkable benefits on exercise tolerance and functional capacity via 6MWT.[51] A similar improvement was achieved in lung cancer patients post lung resection.[52] These improvements can be explained by improved oxygen saturation due to airway secretion clearance utilizing ACBT.[35, 52] However, in one month study of ACBT, it did not lead to improvement in the walking distance. The difference from our study can be explained by the difference in treatment duration.[53]
Threshold PEP also improved the walking distance, which was in agreement with a previous study at which PEP was used with patients with moderate-to-severe COPD. The improvement in walking distance was accompanied by improvements in oxygen saturation during recovery and a decrease in peak exercise heart rate in the PEP group.[54] Even in severe COPD patients who used PEP, there was an improvement in the walking distance during 6MWT in a randomized crossover trial.[55] In contradiction with the current study, another study found only a small improvement obtained by using PEP, but there was no clinical significance regarding the walking distance.[40] Although these improvements were not clinically relevant directly to walking, but may still indicate a correct strategy in improving lung function which may help in executing the walking.
Acapella improved the six-minute walking distance after six months. However, an earlier study on COPD patients found improvement in exercise performance of greater than 10% but not clinically significant.[56]
Quality Of Life (Qol):
Quality of life using COPD assessment test scores showed significant improvements in both the treatment groups B and C and the control group A. All groups test scores decreased. There was no significant difference between the two treatment groups, as well as significant differences between the two treatment groups and the control group.
ACBT showed improvements in earlier studies on the quality of life in mild to moderate asthmatic adult patients.[57] Also, it improved COPD-related symptoms, greatly improving the quality of life in COPD failure patients.[58] This can be explained by an increase in the depth of breathing, muscle strength, and oxygenation. These are mechanisms for reducing dyspnea and improving quality of life.[58]
PEP therapy is associated with improvement in pulmonary function and QOL compared to no treatment,[59, 60] similar to the current study. The improvement can be attributed to increasing sputum expectoration [60] and to the improvement in respiratory functions, cough, and dyspnea.[61] On the other hand, other studies had contradicting evidence to the current study. COPD patients treated with PEP did not have any improvement in respiratory symptoms, quality of life, nor on COPD exacerbations incidence.[62]
Acapella group also showed significant improvements in quality of life in previous studies,[59, 63] as the current study. This was attributed to improvement in sputum expectoration.
Conclusion:
Both treatment groups (PEP, and Acapella) and the control group (ACBT) improved moderate COPD spirometry, walking distance, and quality of life. There was no significant difference between threshold PEP and Acapella. ACBT, which is a routine treatment alone, showed improvements but to a lesser extent than Acapella and PEP.
Acknowledgments:
The authors express their gratitude to physiotherapy colleges in inpatients Cairo University hospitals Kasr el Einy hospitals, Cairo, Egypt.
References
- Boutou AK. Physical Inactivity and Sedentarism among COPD Patients: What About Dynamic Hyperinflation?. COPD. 2019 Feb;16(1):108.
- Brandsma CA, de Vries M, Costa R, Woldhuis RR, Königshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair?. European Respiratory Review. 2017 Dec 31;26(146):170073.
- World Health Organization. Chronic obstructive pulmonary disease (COPD) fact sheet. World Health Organization. http://www. who. int/en/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)[Nov. 2017]. 2017.
- Dhamane, A. D., Moretz, C., Zhou, Y., Burslem, K., Saverno, K., Jain, G., ... & Kaila, S. (2015). COPD exacerbation frequency and its association with health care resource utilization and costs. International journal of chronic obstructive pulmonary disease, 10, 2609.
- Tashkin D. (2019). Heroin Smoking and COPD. Chest, 155(2), pp.247-248.
- AlJohani, K., & AlZahrani, A. S. (2017). Awareness among Medical and Dental Students Regarding the Relationship between Periodontal and Systemic Conditions. International Journal of Pharmaceutical Research & Allied Sciences, 6(4).
- Talari H, Zamani B, Jafari M. Association between bone mineral density (BMD) and carotid artery intima-media thickness (CIMT): a cross sectional study. Journal of Biochemical Technology, 2018; 2: 23-28.
- Alshammari FD. Do Non-Viral Microorganisms Play a Role in the Aetiology of Human Cancers? A Review. International Journal of Pharmaceutical Research & Allied Sciences. 2018 Oct 1;7(4): 179-185.
- National Institute for Health and Care Excellence [Chronic obstructive pulmonary disease in over 16s: diagnosis and management Draft for consultation, February 201], Published in 2019. Website: https://www.nice.org.uk/guidance/gid-ng10128/documents/draft-guideline [Accessed 17 June 2019].
- National Institute for Health and Care Excellence. [Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care], Published in 20 Website: http://guidance.nice.org.uk/CG101/Guidance/pdf/English [Accessed 17 June 2019]
- Laurent GJ, Shapiro SD. (2006). Encyclopedia of respiratory medicine. Amsterdam: Elsevier:332-343.
- Andrews J, Sathe NA, Krishnaswami S, McPheeters ML. Nonpharmacologic airway clearance techniques in hospitalized patients: a systematic review. Respiratory care. 2013 Dec 1;58(12):2160-86.
- Tang CY, Taylor NF, Blackstock FC. Chest physiotherapy for patients admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease (COPD): a systematic review. Physiotherapy. 2010 Mar 1;96(1):1-3.
- Woravutrangkul S, Jarungjitaree S, Sritara C, Vachalathiti R, Chuaychoo B. Efficacy of pursed lips breathing with forced expiration techniques and active cycle of breathing technique on pulmonary mucus clearance in healthy subjects. Journal of Physical Therapy Science. 2010;22(3):247-54.
- Yohannes AM, Connolly MJ. A national survey: percussion, vibration, shaking and active cycle breathing techniques used in patients with acute exacerbations of chronic obstructive pulmonary disease. Physiotherapy. 2007 Jun 1;93(2):110-3.
- Mckoy NA, Wilson LM, Saldanha IJ, Odelola OA, Robinson KA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews. 2016(7).
- Mikelsons C. The role of physiotherapy in the management of COPD. Respiratory Medicine: COPD Update. 2008 Feb 1;4(1):2-7.
- Üzmezoğlu B, Altıay G, Özdemir L, Tuna H, Süt N. The efficacy of flutter® and active cycle of breathing techniques in patients with bronchiectasis: a prospective, randomized, comparative study. Turkish thoracic journal. 2018 Jul;19(3):103.
- Monteiro MB, Berton DC, Moreira MÂ, Menna-Barreto SS, Teixeira PJ. Effects of expiratory positive airway pressure on dynamic hyperinflation during exercise in patients with COPD. Respiratory Care. 2012 Sep 1;57(9):1405-12.
- Lee AL, Denehy L, Wilson JW, Roberts S, Stirling RG, Heine RG, Button BM. Upright positive expiratory pressure therapy and exercise: effects on gastroesophageal reflux in COPD and bronchiectasis. Respiratory care. 2012 Sep 1;57(9):1460-7.
- Lee AL, Burge AT, Holland AE. Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis. Cochrane Database of Systematic Reviews. 2017(9).
- McIlwaine M. Physiotherapy and airway clearance techniques and devices. Paediatric respiratory reviews. 2006 Jan 1;7:S220-2.
- Mueller G, Bersch-Porada I, Koch-Borner S, Raab AM, Jonker M, Baumberger M, Michel F. Laboratory evaluation of four different devices for secretion mobilization: acapella choice, green and blue versus water bottle. Respiratory care. 2014 May 1;59(5):673-7.
- dos Santos AP, Guimarães RC, de Carvalho EM, Gastaldi AC. Mechanical behaviors of Flutter VRP1, Shaker, and Acapella devices. Respiratory care. 2013 Feb 1;58(2):298-304.
- Ni Y, Ding L, Yu Y, Dai R, Chen H, Shi G. Oscillatory positive expiratory pressure treatment in lower respiratory tract infection. Experimental and therapeutic medicine. 2018 Oct 1;16(4):3241-8.
- Syamlal G, Doney B, Mazurek JM. Chronic Obstructive Pulmonary Disease Prevalence Among Adults Who Have Never Smoked, by Industry and Occupation—United States, 2013–2017. Morbidity and Mortality Weekly Report. 2019 Apr 5;68(13):303.
- Tageldin MA, Nafti S, Khan JA, Nejjari C, Beji M, Mahboub B, Obeidat NM, Uzaslan E, Sayiner A, Wali S, Rashid N. Distribution of COPD-related symptoms in the Middle East and North Africa: results of the BREATHE study. Respiratory medicine. 2012 Dec 1;106:S25-32.
- Hanson C, Rutten EP, Wouters EF, Rennard S. Influence of diet and obesity on COPD development and outcomes. International journal of chronic obstructive pulmonary disease. 2014;9:723.
- Rasslan Z, Stirbulov R, Junior RS, Curia ST, da Conceição Lima CA, Perez EA, Oliveira EF, Donner CF, Oliveira LV. The impact of abdominal adiposity measured by sonography on the pulmonary function of pre-menopausal females. Multidisciplinary respiratory medicine. 2015 Dec;10(1):23.
- Gea J, Pascual S, Casadevall C, Orozco-Levi M, Barreiro E. Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings. Journal of thoracic disease. 2015 Oct;7(10):E418.
- Hallin R, Gudmundsson G, Ulrik CS, Nieminen MM, Gislason T, Lindberg E, Brøndum E, Aine T, Bakke P, Janson C. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respiratory medicine. 2007 Sep 1;101(9):1954-60.
- Ringbaekl TJ, Viskuml K, Lange P. BMI and oral glucocorticoids as predictors of prognosis in COPD patients on long-term oxygen therapy. Chronic respiratory disease. 2004 Apr;1(2):71-8.
- Eaton T, Young P, Zeng I, Kolbe J. A randomized evaluation of the acute efficacy, acceptability and tolerability of flutter and active cycle of breathing with and without postural drainage in non-cystic fibrosis bronchiectasis. Chronic Respiratory Disease. 2007 Feb;4(1):23-30.
- Kumar M, Kumaran P, Kumar T, Resan A, Thi G.. Impact of pursed lip breathing in improving exercisenduranceIn non-spontaneous pursed lip breathing chronic obstructive pulmonary disease patients. International Journal of Pharma and Bio Sciences, 2017; 8(3): 111.
- Savci S, Ince DI, Arikan H. A comparison of autogenic drainage and the active cycle of breathing techniques in patients with chronic obstructive pulmonary diseases. Journal of Cardiopulmonary Rehabilitation and Prevention. 2000 Jan 1;20(1):37-43.
- Pryor C. 1999 Review of Recruitment Incentives for Allied Health Care Professionals. Hospital Pharmacy. 1999 Nov;34(11):1307-10.
- Fatima SS, Rehman R, Saifullah, Khan Y. Physical activity and its effect on forced expiratory volume. Journal of the Pakistan Medical Association, 2019; 3(2): 310-312.
- Patterson JE, Bradley JM, Elbom JS. Airway clearance in bronchiectasis: a randomized crossover trial of active cycle of breathing techniques (incorporating postural drainage and vibration) versus test of incremental respiratory endurance. Chronic respiratory disease. 2004 Jul;1(3):127-30.
- Christensen EF, Nedergaard T, Dahl R. Long-term treatment of chronic bronchitis with positive expiratory pressure mask and chest physiotherapy. Chest. 1990 Mar 1;97(3):645-50.
- Gass R, Merola P, Monteiro MB, Cardoso DM, Paiva DN, Teixeira PJ, Knorst MM, Berton DC. Effects of Expiratory Positive Airway Pressure on Exercise Tolerance, Dynamic Hyperinflation, and Dyspnea in COPD. Respiratory care. 2017 Oct 1;62(10):1298-306.
- O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2006 Apr;3(2):180-4.
- Padkao T, Boonsawat W, Jones CU. Conical-PEP is safe, reduces lung hyperinflation and contributes to improved exercise endurance in patients with COPD: a randomised cross-over trial. Journal of physiotherapy. 2010 Jan 1;56(1):33-9.
- Narula D, Nangia V. Use of an oscillatory PEP device to enhance bronchial hygiene in a patient of post-H1NI pneumonia and acute respiratory distress syndrome with pneumothorax. Case Reports. 2014 Mar 7;2014:bcr2013202598.
- Vreys M, Proesmans M, De Boeck K. 361 Cleaning a Positive Expiratory Pressure (PEP) mask: an investigation of patient routines. Journal of Cystic Fibrosis. 2006 Jan 1;5:S80.
- Patterson JE, Hewitt O, Kent L, Bradbury I, Elborn JS, Bradley JM. Acapella® versususual airway clearance'during acute exacerbation in bronchiectasis: a randomized crossover trial. Chronic Respiratory Disease. 2007 May;4(2):67-74.
- Evangelodimou A, Grammatopoulou E, Skordilis E, Haniotou A. The Effect of Diaphragmatic Breathing on Dyspnea and Exercise Tolerance During Exercise in COPD Patients. Chest. 2015 Oct 1;148(4):704A.
- Jayson CJ, Vaishnavi G. To compare the effects of acapella and diaphragmatic breathing exercises in copd patients. International Journal of Current Research, 2018; 10(1): 112.
- McIlwaine M, Bradley J, Elborn JS, Moran F. Personalising airway clearance in chronic lung disease. European Respiratory Review. 2017 Mar 31;26(143):160086.
- Suggett J, Meyer A. A Laboratory Assessment Into the Efficiency and Effectiveness of Different Oscillating Positive Expiratory Pressure Devices by Means of Patient Simulated Expiratory Waveforms. Chest. 2017 Oct 1;152(4):A970.
- Mueller G, Bersch-Porada I, Koch-Borner S, Raab AM, Jonker M, Baumberger M, Michel F. Laboratory evaluation of four different devices for secretion mobilization: acapella choice, green and blue versus water bottle. Respiratory care. 2014 May 1;59(5):673-7.
- Elsayed SH, Basset WK, Fathy KA. Impact of Active Cycle of Breathing Technique on Functional Capacity in Patient With Bronchiectasis. International Journal of Therapies and Rehabilitation Research. 2015;4(5):287.
- Yang M, Zhang JE, Huang XX, Li CZ, Hong ZX, Zhang SW. Effect of the self-efficacy-enhancing active cycle of breathing technique on lung cancer patients with lung resection: A quasi-experimental trial. European Journal of Oncology Nursing. 2018 Jun 1;34:1-7.
- Cesario A, Ferri L, Galetta D, Pasqua F, Bonassi S, Clini E, Biscione G, Cardaci V, Di Toro S, Zarzana A, Margaritora S. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung cancer. 2007 Aug 1;57(2):175-80.
- Nicolini A, Merliak F, Barlascini C. Use of positive expiratory pressure during six minute walk test: results in patients with moderate to severe chronic obstructive pulmonary disease. Multidisciplinary respiratory medicine. 2013 Dec;8(1):19.
- Russo D, Simonelli C, Paneroni M, Saleri M, Piroddi IM, Cardinale F, Vitacca M, Nicolini A. ¿ Cuál es el nivel óptimo de presión espiratoria positiva (PEP) capaz de mejorar la tolerancia a la deambulación de los pacientes con enfermedad pulmonar obstructiva cronica (EPOC) grave?. Archivos de Bronconeumología. 2016 Jul 1;52(7):354-60.
- West K, Wallen M, Follett J. Acapella vs. PEP mask therapy: a randomised trial in children with cystic fibrosis during respiratory exacerbation. Physiotherapy theory and practice. 2010 Jan 1;26(3):143-9.
- Sundus S, Memoona S, Muhammad IN, Rashid HN. Effect of Active Cycle of Breathing Technique in Adult Asthmatic Patients in Pakistan. Asian Journal of Medicine and Biomedicine. 2017 Dec 21;1(1):32-7.
- Muselema CK, Jere M, Chongwe G, Goma FM. Pulmonary Function Responses to Active Cycle Breathing Techniques in Heart Failure Patients at the University Teaching Hospital (UTH), Lusaka, Zambia. Medical Journal of Zambia. 2015;42(2):47-53.
- Lee AL, Williamson HC, Lorensini S, Spencer LM. The effects of oscillating positive expiratory pressure therapy in adults with stable non-cystic fibrosis bronchiectasis: a systematic review. Chronic respiratory disease. 2015 Feb;12(1):36-46.
- Bellone A, Spagnolatti L, Massobrio M, Bellei E, Vinciguerra R, Barbieri A, Iori E, Bendinelli S, Nava S. Short-term effects of expiration under positive pressure in patients with acute exacerbation of chronic obstructive pulmonary disease and mild acidosis requiring non-invasive positive pressure ventilation. Intensive care medicine. 2002 May 1;28(5):581-5.
- Mascardi V, Grecchi B, Barlascini C, Banfi P, Nicolini A. Effectiveness of temporary positive expiratory pressure (T-PEP) at home and at hospital in patients with severe chronic obstructive pulmonary disease. Journal of thoracic disease. 2016 Oct;8(10):2895.
- Osadnik CR, McDonald CF, Miller BR, Hill CJ, Tarrant B, Steward R, Chao C, Stodden N, Oliveira CC, Gagliardi N, Holland AE. The effect of positive expiratory pressure (PEP) therapy on symptoms, quality of life and incidence of re-exacerbation in patients with acute exacerbations of chronic obstructive pulmonary disease: a multicentre, randomised controlled trial. Thorax. 2014 Feb 1;69(2):137-43.
- Bonilha AG, Onofre F, Vieira ML, Prado MY, Martinez JA. Effects of singing classes on pulmonary function and quality of life of COPD patients. International journal of chronic obstructive pulmonary disease. 2009;4:1.
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]