The effects of Phentermine and Lorcaserin on body weight, food intake, and visceral fat in Mice: comparative study
Nibras J Tahseen, Nada S Shaker, Zeena A Hussein*, Ahmed F Mutee, Ahmed M Al-Tuhafi
Al-Rasheed University College, Pharmacy Department, Baghdad, Iraq.
Correspondence: Zeena A Hussein; Al-Rasheed University College, Pharmacy Department, Baghdad, Iraq. E-mail: zeena.kanada @ yahoo.com
|
ABSTRACT Phentermine and lorcaserin are two commonly-used pharmacological agents to address the growing pandemic of obesity and overweight. The two drugs have different modes of action and duration of use. The aim of the current study was to assess their effects on food intake, weight loss, and visceral fat in normal lab mice. Thirty white albino mice were recruited in the study. They were divided into 2 groups; the phentermine treated group (Ph. Group) consisted of 10 animals that received daily single oral dose of 0.3 mg/kg of phentermine for 4 weeks and the lorcaserin treated group (Lo. Group) consisted of 20 animals that received daily single oral dose of 0.2 mg/kg of lorcaserin. Ten of these mice were sacrificed after 4 weeks of treatment alongside the Ph. Group. The other 10 animals continued treatment for another 4 weeks (total 8 weeks) and then were sacrificed. Study parameters included body weight (g), food intake (g/animal/day), percentage of changes in these two parameters, epididymal fat pad weight and its histomorphometry changes. Body weight and daily food consumption dropped considerably in animals treated with both drugs. The drop was more significant at a shorter period (half time) in phentermine-treated animals than in lorcaserin-treated ones. The histomorphometry changes reflect the histo-physiological characteristics of visceral fat as a preferential target for weight loss. Phentermine can increase body weight and visceral fat changes in half of the time of that of lorcaserin but the final maintained results and lesser adverse effects may be in favor of lorcaserin. The combination of the two drugs may offer a better understanding of their combined work and require further study. Keywords: Phentermine, Lorcaserin, Appetite suppression, Body weight, Visceral fat |
Introduction
Overweight/obesity is a common problem in the modern age. Dieting and exercise alone may be inefficient in losing weight and maintaining a healthy risk-free weight goal. Modern lifestyle and inappropriate food intake (in quantity and quality) are the main factors to blame [1]. However, weight loss achieved by lifestyle modifications alone is often limited and difficult to maintain. Increased energy expenditure and reduced caloric intake are neutralized by adaptive physiologic responses [2]. Anti-obesity pharmacotherapy is one strategy to offset the adaptive changes in appetite and energy expenditure that occur with weight loss and to improve adherence to lifestyle interventions [3]. Medication for obesity can be considered if patients have a body mass index (BMI) of >30 kg/m2, or >27 kg/m2 with weight-related comorbidities, such as hypertension, dyslipidemia, obstructive sleep apnea, and type 2 diabetes [4, 5]. Since obesity is a chronic disease, most antiobesity medications have been approved for long-term treatment. Until a few years ago, orlistat and phentermine (and other sympathomimetic amines) were the only FDA-approved antiobesity drugs. In 2012, phentermine/topiramate extended-release (ER) and lorcaserin were approved; in 2014, naltrexone sustained-release (SR)/bupropion SR and liraglutide 3.0 mg were approved [6].
Phentermine is an adrenergic agonist that increases resting energy expenditure and suppresses appetite. Phentermine is indicated for using in a short-term (three months), as there are no long-term safety trials of phentermine monotherapy; but it was approved in combination with topiramate ER for long-term therapy. Many physicians prescribe phentermine for more than three months as an off-label therapy for continuous management of weight. In 2012, the FDA approved phentermine/topiramate ER for chronic management of weight as an adjunct to increased physical activity and decreased-calorie diet. The rationale for a combination medication is that appetite regulation involves multiple pathways, so targeting different mechanisms simultaneously can have an additive impact on body weight [7].
Lorcaserin, selective serotonin (5-hydroxytryptamine [5HT])-2C receptor agonist, was approved by the FDA in 2012 as a long-term treatment of obesity. Lorcaserin reduces appetite and increases satiety by binding to the 5HT-2C receptors on anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. Lorcaserin was designed to avoid cardiac valvular effects mediated through the 5HT-2B receptor because of its selective agonism of the serotonin 2C receptor [8]. Due to the possible abuse of both drugs by non-eligible candidates for accelerated weight loss, the current study was designed to examine and compare the effects of drugs in healthy non-obese mice over different durations.
Materials and Methods:
Materials:
Phentermine was purchased as ADIPEX-P® (37.5 mg phentermine hydrochloride tablets containing 30 mg phentermine base) from PlivaHrvatska (Croatia). Lorcaserin was purchased as BELVIQ® (10 mg lorcaserin hydrochloride tablets) from Aena Pharmaceuticals (Switzerland).
Laboratory Animals and Grouping:
The study was conducted at the labs of Al-Rasheed University College, Pharmacy Department (Baghdad – Iraq) after obtaining animal ethical approval. Thirty adult male albino mice were used from the animal house at the college. They had an average age of 12 weeks and the weight range of 21-22.9 g (22.17±0.54). The animals were divided into 2 groups: The phentermine-treated group (Ph. Group) consisted of 10 animals that daily received a single oral dose of 0.3 mg/kg of phentermine for 4 weeks. The lorcaserin-treated group (Lo. Group) consisted of 20 animals that daily received a single oral dose of 0.2 mg/kg of lorcaserin. Ten of these mice were sacrificed after 4 weeks of treatment alongside the Ph. Group. The other 10 animals continued treatment for another 4 weeks (total 8 weeks) and then were sacrificed.
Preparing and Administering the Required Dose:
The drug tablet was pulverized using a Mortar Grinder (Retsch® RM 200, Germany) then emulsified by Tween 80 and diluted with distilled water. The dose was calculated for each animal and was given orally by oral gavage using a flexible oral feeding needle. The animal dose was calculated depending on the body surface area equations in equivalence to human approved dose [9].
Measurement of Daily Feed Intake:
The animals were kept in individual cages for one week before the drug treatment to adapt to lab conditions and allow for daily food intake estimation. Once treatment began, they were weighed daily and provided with 10 grams/day of regular rodent chow (Teklad® 4% Fat Mouse/Rat Diet, Harlan Laboratories, Seoul-Korea). At 8:00 AM every morning, the remaining food pellets were collected and weighed to calculate food intake. Each animal was weighed daily, and at the end of the week, the amount of weight loss was calculated [10].
Histopathological Examination:
At the end of the treatment duration for each group, mice were sacrificed under anesthesia and the right epididymal visceral fat pad was dissected, weighed, and prepared for histological examination to study changes in visceral fat tissue. Histological sections of 5πm thickness were stained with H&E and examined using a light microscope with a built-in camera (Leica® DM4000 B LED). Five fields were examined in each slide and the diameter of adipocytes was measured using Image-J software after digital image scaling.
Statistical Analysis:
Using the analysis of variance (SPSS Software, version 20.0), the results were statistically analyzed, and whenever there was a difference between the correlated groups, student’s t-test was performed to estimate the significance degree by comparing the mean of data and standard deviation of each group. Therefore, data are presented as mean ± standard deviation, at a 95% confidence interval (P ≥ 0.05).
Results:
There was not any significant difference in the initial body weight between the 2 groups, but the drop-in body weight occurred at a statistically significant higher rate in the Ph. Group than in the Lo. Group from week-1 to week-4 (Figure 1). There was no significant difference in the final body weight in the Lo. group (after 8 weeks) when compared to the final body weight in the Ph. Group (after 4 weeks). Similarly, the change percentage in body weight was statistically greater in Ph. Group than in the Lo. Group by the end of week 4. However, the change percentage in body weight in the Lo. Group became significantly greater by the end of week-8 (Figure 2).
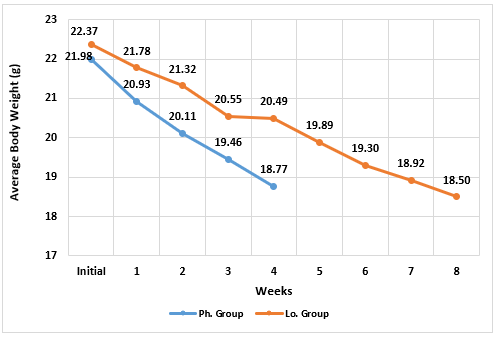
Figure 1: Changes in body weight (grams) in animals receiving phentermine for 4 weeks (Ph. group) and lorcaserin (Lo. group) for 8 weeks.
(Data presented as means, *= statistically significant difference with P-value <0.05)
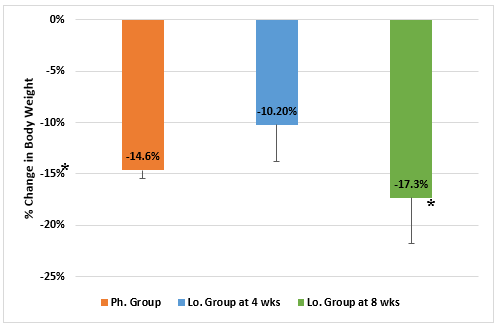
Figure 2: Change percentage in body weight in animals receiving phentermine for 4 weeks (Ph. group) and lorcaserin (Lo. group) for 4 & 8 weeks.
(Data presented as Mean ± SD, *= statistically significant difference with P-value <0.05)
The initial food consumption was almost the same in the two groups. A statistically significant drop in daily food consumption became more apparent in the Ph. Group than in the Lo. group by the 2nd week of treatment and continued to be greater during the third & fourth weeks (Figure 3). Moreover, the Lo. group food consumption showed a slight increase during the fourth week. By the end of the 4th week, the change percentage in food consumption was significantly greater in the Ph. group and remained so for the Lo. group animals than continued treatment for 8 weeks (Figure 4).
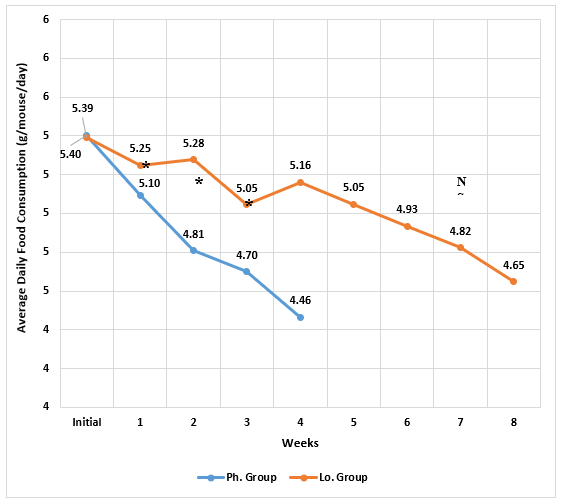
Figure 3: The change in daily food consumption (g/mouse/day) of animals receiving phentermine for 4 weeks (Ph. group) and lorcaserin (Lo. group) for 8 weeks.
(Data presented as means, *= statistically significant difference with P-value <0.05)
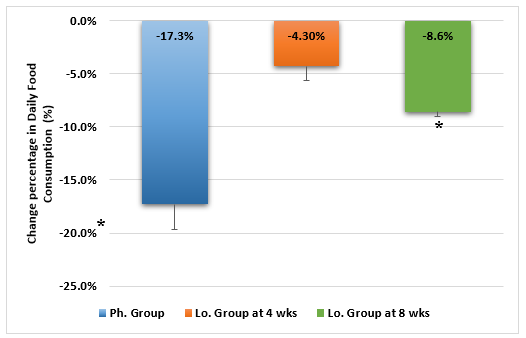
Figure 4: Change percentage in daily food consumption of animals receiving phentermine for 4 weeks (Ph. group) and lorcaserin (Lo. group) for 4 & 8 weeks.
(Data presented as mean ± SD, *= statistically significant difference with P-value <0.05)
The epididymal fat pad showed a statistically significant difference among the sacrificed animals. It had the lowest weight in the Ph. group and the highest weight in the Lo. group animals sacrificed at 4 weeks. Lo. group animals, sacrificed at 8 weeks, showed significant differences but somewhere intermediate weights (Figure 5).
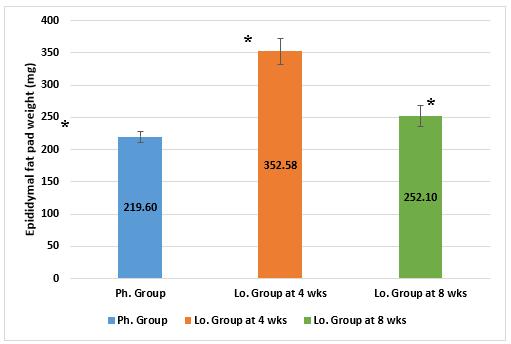
Figure 5: Epididymal fat pad weights (mg) in animals receiving phentermine for 4 weeks (Ph. group) and lorcaserin (Lo. group) for 4 & 8 weeks.
(Data presented as mean ± SD, *= statistically significant difference with P-value <0.05)
The histomorphometry measurement of epididymal fat cell diameter showed similar results. Adipocyte diameter showed a statistically significant difference among the sacrificed animals. It had the lowest values in the Ph. group and highest values in the Lo. group animals sacrificed at 4 weeks. Lo. group animals sacrificed at 8 weeks showed significantly different but somewhere intermediate values (Figure 6).
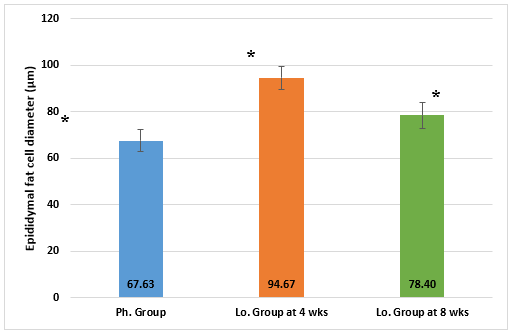 Figure 6: Epididymal adipocyte diameter (µm) in animals receiving phentermine for 4 weeks (Ph. group) and lorcaserin (Lo. group) for 4 & 8 weeks.
Figure 6: Epididymal adipocyte diameter (µm) in animals receiving phentermine for 4 weeks (Ph. group) and lorcaserin (Lo. group) for 4 & 8 weeks.
(Data presented as mean ± SD, *= statistically significant difference with P-value <0.05).
The histological examination of epididymal fat pad sections in Ph. group animals revealed mostly small-to-moderate sized, mostly rounded adipocytes with prominent connective tissue septa and engorged blood vessels (Figure 7). In Lo. group animals sacrificed at 4 weeks, adipocytes were mostly large-sized, round or polygonal with no clear septa. In Lo. group animals sacrificed at 8 weeks, the cells had mostly moderate size with clear connective tissue septa.
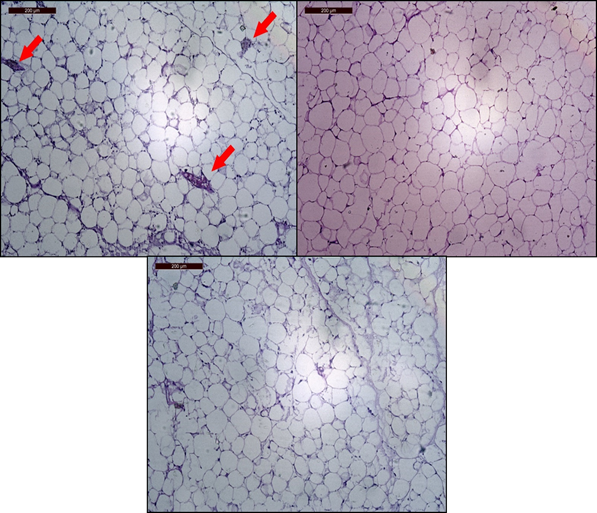
Figure 7: Epididymal fat sections stained with H&E (100X). Upper left: Animals receiving phentermine for 4 weeks (Ph. group) showing small-to-moderate sized fat cells with clear connective tissue septa and engorged blood vessels (red arrows). Upper right: Animals receiving lorcaserin (Lo. group) for 4 weeks showing mostly large round and polygonal fat cells. Lower center: Animals receiving lorcaserin (Lo. group) for 8 weeks showing moderate-sized fat cells with clear connective tissue septa.
Discussion:
Although the cornerstone of weight loss is caloric restriction and increased energy expenditure, it is commonly seen now that these measures alone are not enough to maintain a healthy weight and pharmacologic aid has become increasingly used to achieve such a goal. Phentermine and lorcaserin are two commonly used drugs for such purposes [11]. The current study showed that both drugs can lower food intake and body weight, but the effects achieved by phentermine over the short term exceed the effects of lorcaserin over the same period but maybe as equivalent when lorcaserin is used in a long term. Phentermine (when used alone) is a noradrenergic sympathomimetic drug that acts centrally to suppress appetite by up-regulating certain messenger activities like serotonin, noradrenaline, and dopamine [12]. Some researchers proposed that it might also increase energy expenditure by hypothalamic stimulation of the sympathetic autonomic nervous system [13]. The problem with using phentermine alone is its potential cardiovascular adverse effects such as palpitations, tachycardia, and hypertension and on long-term possible valvular heart disease and pulmonary hypertension [14]. The introduction of the phentermine/topiramate combination allowed a greater amount of weight loss over a shorter period, which in turn reduced the incidence of cardiovascular and neurological side effects [15]. The current study used phentermine monotherapy since it was only used for a short period.
Unlike phentermine, there are few papers in the literature reporting the actions of lorcaserin in pre-clinical models of obesity and feeding [16], and the forewarning to its approval required the need to perform post-marketing studies [17]. Lorcaserin is a 5-hydroxytryptamine (5-HT2C) agonist with a high affinity that stimulates 5-HT2C receptors on the pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus; this leads to the release of an alpha-melanocortin-stimulating hormone (alpha-MSH) that acts on melanocortin-4 receptors in the paraventricular nucleus to suppress appetite [18]. Lorcaserin is indicated in combination with increased physical activity and decreased-calorie diet for chronic weight management in obesity since short-term use of high doses can cause 5-HT2C-related neurological side effects [19]. Although lorcaserin-related neuronal networks can affect energy balance [20], there is no confirmed evidence of such an effect of the drug. This may explain the doubled duration lorcaserin needed to achieve weight loss compared to phentermine in the current study since phentermine has a double mode of action. It may also explain the dose-related increase in food consumption after a few weeks of use, possibly due to physiological receptor down-regulation.
The study used epididymal fat as a depot of visceral fat in reference to subcutaneous fat due to histo-physiological properties of visceral fat in terms of weight loss. Visceral fat is more susceptible to fat depot loss because it is more metabolically active than subcutaneous fat possibly due to more cellularity, greater vascularization, more innervation, and greater susceptibility to certain hormones [21]. This is supported by the result of engorged blood vessels in Ph. group fat pads due to the high circulation of lost fats to the circulation. The presence of clear connective tissue septa also reflects the reduction in adipocyte size hindering the cells from abutting each other and the septa [22].
Conclusion:
Phentermine can change body weight and visceral fat in half the time of that of lorcaserin but the final maintained results and lesser adverse effects may be in favor of lorcaserin. The combination of the two drugs may offer a better understanding of their combined work and requires further study.
Conflict of interest:
All authors declare that there is no conflict of interest among the authors.
References
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences. 2013 Apr 2;110(14):5695-700.
- Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. New England Journal of Medicine. 2011 Oct 27;365(17):1597-604.
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. International journal of obesity. 2015 Aug;39(8):1188.
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American college of cardiology. 2014 Jul 1;63(25 Part B):2985-3023.
- Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2015 Feb 1;100(2):342-62.
- Saunders KH, Umashanker D, Igel LI, Kumar RB, Aronne LJ. Obesity pharmacotherapy. Medical Clinics. 2018 Jan 1;102(1):135-48.
- Haslam D. Weight management in obesity–past and present. International journal of clinical practice. 2016 Mar;70(3):206-1
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. New England Journal of Medicine. 2010 Jul 15;363(3):245-56.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. Journal of basic and clinical pharmacy. 2016 Mar;7(2):27-31.
- Helland SJ, Grisdale-Helland B, Nerland S. A simple method for the measurement of daily feed intake of groups of fish in tanks. Aquaculture. 1996 Jan 1;139(1-2):157-63.
- Smith SR, Garvey WT, Greenway FL, Zhou S, Fain R, Pilson R, Fujioka K, Aronne LJ. Coadministration of lorcaserin and phentermine for weight management: A 12‐week, randomized, pilot safety study. Obesity. 2017 May;25(5):857-65.
- Sweeting AN, Tabet E, Caterson ID, Markovic TP. Management of obesity and cardiometabolic risk–role of phentermine/extended release topiramate. Diabetes, metabolic syndrome and obesity: targets and therapy. 2014;7:35-44.
- Mathew H, Paschou SA, Aramapatzi KM, Hsu W, Mantzoros CS. Obesity: genetics, pathogenesis, therapy. Principles of Diabetes Mellitus. 2017:1-7.
- Daneschvar HL, Aronson MD, Smetana GW. FDA-approved anti-obesity drugs in the United States. The American journal of medicine. 2016 Aug 1;129(8):879-e1.
- Thompson JA, Heaton PC, Kelton CM. Efficacy Of Phentermine Monotherapy, Topiramate Monotherapy, And Phentermine/Topiramate Combination Therapy On Weight Loss: A Network Meta-Analysis Of Randomized Controlled Trial Data. Value in Health. 2014 May 1;17(3):A224.
- Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D. Discovery and structure− activity relationship of (1 R)-8-chloro-2, 3, 4, 5-tetrahydro-1-methyl-1 H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. Journal of medicinal chemistry. 2007 Dec 21;51(2):305-13.
- Higgs S, Cooper AJ, Barnes NM. The 5-HT 2C receptor agonist, lorcaserin, and the 5-HT 6 receptor antagonist, SB-742457, promote satiety; a microstructural analysis of feeding behaviour. Psychopharmacology. 2016 Feb 1;233(3):417-24.
- Gustafson A, King C, Rey JA. Lorcaserin (Belviq): A selective serotonin 5-HT2C agonist in the treatment of obesity. Pharmacy and Therapeutics. 2013 Sep;38(9):525-34.
- FDA Center for Drug Evaluation and Research Belviq NDA 022529 drug label, June 27, 2012. Available at: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm . Accessed September 29, 2017.
- Burke LK, Doslikova B, D'Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, de Morentin PB, Ogunnowo-Bada E, Cansell C, Valencia-Torres L, Garfield AS. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Molecular metabolism. 2016 Mar 1;5(3):245-52.
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity reviews. 2010 Jan;11(1):11-8.
- Rehfeld A, Nylander M, Karnov K. Compendium of Histology: A Theoretical and Practical Guide. Springer; 2017 Sep 7. (pp. 201-207). Springer, Cham.
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]