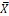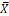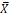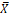Influence of PMF on RBCs and therapeutic action of vitamins
Manal B. Abd El-Fatah1*, Soheir Sh. Rezk–Allah2, Ragia M. Kamel2, Neveen Abd El-Latif Abd El-Raoof2, Nadia K. Maree3
1Physiotherapist at BeniSuef Specialized hospital, BeniSuif.2Professor of Physical Therapy, Basic Science Department, Faculty of Physical Therapy, Cairo University, Cairo, Egypt.3Professor of Internal Medicine, Faculty of Medicine, Al Azhar University, Cairo, Egypt.
Correspondence:Manal B. Abd El-Fatah. Physiotherapist at Beni Suef Specialized hospital, BeniSuif./ ElManial, Cairo 11562, Egypt.
Email: manal.bakry2000 @ gmail.com
|
ABSTRACT
Background: Magnetic fields are considered as one of the most developing and common used modalities in the field of physical therapy. Physical therapists use magnetic fields for the treatment of various musculoskeletal disorders such as back dysfunction. Pulsed magnetic field (PMF) was reported to have hazardous effects besides the beneficial effects to subjects who are subjected to it for treatment or even therapists who are present with patients at the PMF Purposes: To investigate the efficacy of PMF on RBC morphology and investigate the treatment action of vitamins E and C versus injury influences induced by PMF. Subject: sixty patients (males and females) with mechanical low back pain (MLBP) were referred by their physician to receive PMF for treatment of their back pain; their ages ranged from 20-40 years; they were classified randomly into four equal groups. Method: Patients in group I were treated with PMF (the frequency of 50 Hz, the intensity of 20 gausses, and duration of 20 min) and traditional physical therapy program. Patients in group II received the traditional physical therapy program only. Patients in group III were treated with PMF and traditional physical therapy program followed by taking vitamin C, and patients in group VI were treated with PMF and traditional physical therapy program followed by taking vitamin E. The treatment was applied 3 sessions per week for four successive weeks. The Red Blood Cells (RBCs) count, hemoglobin (HB), Packed Cell Volume (PCV) and Mean Corpuscular Volume (MCV) were measured before and after 4 weeks of treatment. Results: There was a significant improvement of RBC morphology after treatments in the groups, treated with PEMF in addition to vitamin C or E. Conclusion: The RBC morphology is influenced by PMF exposure. Meanwhile, this alternative showed the signs of advancements with vitamins E and C therapies than PMF exposure alone. Keywords: Pulsed magnetic field, red blood cell, therapeutic effect, vitamins E and C. |
Introduction
Definition of an electromagnetic field is a physical field produced by in motion electrically charged object, which influences the conduct of charged objects in the proximity of the field. The electromagnetic field develops indefinitely everywhere and characterizes the electromagnetic interaction [1].
The magnetic treatment supplies a non-invasive, safe, and easy method to immediately cure the site of damage, the source of suffering and inflammation, and other kinds of diseases. It is now generally desirable to qualify the magnetic fields for various therapeutic processes including late fractures, suffering relief, multiple sclerosis, and Parkinson’s disease. Millions of people worldwide have received assistance in the therapy of the musculoskeletal system, in additional suffering relief [2].
There are many various types of magnetic fields treatments like static magnetic fields (SMF) and pulsed magnetic fields (PMF); each has a great group of frequencies and powers. The scientific studies showed that the small, frequent exposure to pulsed magnetic fields is the maximum influential form of magnetic treatment [3].
PMF therapy increases the permeability of the cell membrane, improves blood circulation, increases oxygen supply, increases ATP production, stimulates healing process and recovery of the injured tissues as well as its analgesic and anti-inflammatory effects [4].
The significant risk electromagnetic waves are common types of serious contaminations probably due to the PMF, which influence the role of the body cells [5]. Electromagnetic waves may cause imponderability in the cell force, which distributes cell action. These problems happen according to the severity of the disruption and damage [6].
In the last few decades, various studies about the biological effect of PMF exposure have been in progress and most of them, carried out on mice, rat, and human showed that PMF induces changes in hematological parameters in these organisms [7].
Blood and blood parameters are the fundamental particles, exposed to PMF. Blood being ions are possible to interact with induced PMF produced by PMF charges. Some reports indicated that PMF might affect some blood parameters of mammalian cells depending on the power densities but others are on the contrary. Several hematological parameters are sensitive to PMF exposure in animals and humans [8].
Epidemiological research, conducted up to now, showed a relationship between the rise of cancer and exposure to PMF. In response to the general concern about the health effects of PMF exposure, the International PMF Project was planned by WHO and the Radiation and Environmental Health Unit, which arranged research on EMF proportional to the Environmental Health Criteria, whose major target was to review the scientific research on the biological influences of exposure to PMF fields to estimate any health dangers due to exposure to these fields. The International Agency for Research on Cancer (IARC) complemented that PMF is probably carcinogenic to humans [9].
In a study by Forgacs et al., 2005 [10] the results reported that the PMF exposure may slightly, but statistically significantly change variations in some hematological measurements of male mice within the physiological domain. It can be seen that though a lot of studies were conducted most especially on the way towards having a clear understanding of the effect of PMF exposure, this issue is still a subject of debate. It is agreed that the basic mechanism of damage to tissues in the body involves free radicals. Living microorganisms naturally preserve an electric charge in their membranes that are fundamental for the normal function of human tissues. These accusations are sensitive to PMF [8].
Some investigators suggest that DNA damage has an oxidative stress mechanism of free radicals induced by PMF [11-13]. The definition of an antioxidant system is body defense techniques that can significantly interact with free radicals and terminate the chain reaction before the deterioration of vital molecules like DNA. When free radical output overtakes antioxidant activity, the oxidative stress occurs. Moreover, frequently long-period exposure to PMF can lead to an excessive increase in the free radical output and induces oxidative stress [14,15].
Studies have shown potential effects of antioxidants, such as vitamin C, vitamin E, and melatonin, on the oxidative stress status induced PMF in animals [16].
Abd-Elaziz et al., 2010 [17] found that there are signs of improvement in the body weight rate and the hematological measurements through therapies with a magnetic field along with vitamins E and C.
The necessity for this research was to progress from the shortage in the quantitative information and knowledge in the published research around the effect of PMF on RBCs’ morphology. This study was designed to provide a guideline about the effects of the pulsed magnetic field on RBCs morphology and to provide a guideline about the therapeutic action of vitamin E and C.
Materials and Methods
The current study was conducted in the out-clinic of Faculty of Physical Therapy, Cairo University. This study was conducted to investigate the efficacy of PMF on RBC morphology and investigate the therapeutic action of vitamins E and C against the harmful effects induced by PMF.
Power of the study:
The power of the study was measured post hoc by G*Power 3.1 software, with the sample size 60 subjects, 0.05 type I error (2 tailed) and effect size of 0.99. The power was 0.98.
Design of study
A Randomized Controlled Trial (RCT) to investigate the efficacy of PMF on RBC morphology and investigate the therapeutic action of vitamins E and C against the harmful effects induced by PMF.
Subjects
A sample of sixty patients, suffering from MLBP for more than 3 months, diagnosed as MLBP by an orthopedist was randomly assigned using a random sequence generator to one of the four study groups, concealed allocation by opaque sealed envelopes. The subjects were referred by a physician or an orthopedist. The study was validated by the Faculty of Physical Therapy ethical committee, Cairo University. All subjects provided written informed consent. The subjects were included if their age ranged from 20-40 years, both male and female.
The patients were randomly classified into four equal groups; each group included fifteen patients:
Group 1: Magnet on Group: 15 patients (8 females and 7 males) were treated with PMF therapy and traditional physical therapy program (ultrasound and infrared).
Group 2: Magnet off Group: 15 patients (9 females and 6 males) were received traditional physical therapy program only.
Group 3: (vitamin C group): 15 patients (9 females and 6 males) were treated with PMF and traditional physical therapy program followed by taking vitamin C.
Group 4: (vitamin E group): 15 patients (7 females and 8 males) were treated with PMF and traditional physical therapy program followed by taking vitamin E.
Inclusion Criteria are:
All patients were approximately at the same age from 20 - 40 years old.
All patients had MLBP for at least 3 months.
All patients should be conscious and ambulant.
Exclusive criteria were:
Age less than 20 years old and more than 40 years old.
Patients suffered from unstable cardiovascular conditions.
Smokers.
Pregnant or lactating women.
Contraindications to electromagnetic exposure (Pacemaker, malignancy, terminal or rapidly progressive illness).
Blood samples were collected from the groups, and then CBC was measured at two different times. The first measurement was done at pre-exposure period and the second was done at immediately post-exposure period.
Evaluation instrumentation:
- Tubes of blood sample:
Complete blood count (CBC): Ethylene diaminetetraacetate (EDTA) tubes:
Purple top tubes should be not overfilled, 50-60 % is suitable.
The specimen was gently mixed by 5-10 times inverting and placed on a rocker for half an hour, then placed at 2-8 ˚C to keep the CBC stable for 24 h.
hemolyzed or Clotted specimens were not acceptable. The clots were checked by a clean wooden applicator stick and gently swirling blood in the tubes.
In order to overcome the surface tension, EDTA microtainers were shaken 10-15 times within the tube.
- Image analyzer:
SAMICA (scanning and measuring with automatic image contour analysis) product of ELBK GMBH, Germany.
The system was composed of a video camera attached to a computer programmed to calculate the size distribution.
It was possible by this system to get the image of the RBCs’ film electronically after magnification up to 1200 times.
Therapeutic instrumentations:
- Electromagnetic field therapy:
The treatment protocol was achieved by using the electromagnetic unit ASA magnetic field. It has solenoids motorized bed, and an appliance. The appliance was connected to electrical mains supplying 230 V at a frequency of 60 or 50 Hz with earth connection (figure 1). It can generate PMF up to 100 Hz and the intensity varies according to the type of solenoid. The spatial layout and intensity of the magnetic field depend on the type of solenoid.
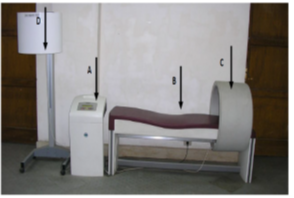
Figure 1: ASA magnetic field (Automatic PMT Quattro pro)
A- The appliance, B- Motorized bed, C- Solenoid, D- Accessory for transcranial application
- Ultrasound:
Using Ultrasound device EnrafNonius – Model: Sonopuls 490U [Made in the Netherlands].
- Infrared radiation:
Infrared was used as a form of heat for many purposes. Its model was 4004/2N. The device had a power of 400 w, voltage 203 v, and frequency of 50/60 Hz.
Procedures:
- Preparation of the subjects:
Each patient of all groups was asked to fill out the information sheet.
Every subject was informed about the measurement.
The experimental protocol was explained in details for every patient before starting the initial assessment.
A written consent form was signed by each patient before starting.
The treated patients were instructed to report any side effects during the treatment sessions.
- Measurement procedures:
The patients were assessed just before and just after the treatment sessions. The assessment procedures included the following items:
CBC
RBCs morphology
The blood was drawn in a test tube containing an anticoagulant (EDTA, sometimes citrate) to stop it from clotting as recommended by Dacie and Lewis 2006 [18].
- Therapeutic procedures:
Procedures of PMF:
EMF was applied once daily, three times per week every day for one month.
Each session was conducted for 20 minutes over the lower lumbar region with the patient placed in a comfortable supine position.
PMF, the frequency of 50 Hz, intensity of 20 gausses and duration of 20 minutes [19].
Procedures of Ultrasound:
The patients were relaxed in a prone position and back free from clothes. It was done in the lower back paraspinal muscles at the maximum tender area. The output frequency set at 1 Hz, continuous mode of application 1.5w/cm2 duration of treatment for 5 minutes/session, 3 sessions/week every other day for one month [20].
Procedures of infrared:
Superficial heating (infrared lamp) for 20 minutes/session at a distance of 60 cm from the lumbar region, while patients in a prone lying position for 12 sessions 3 sessions/week every other day for one month [21].
Each patient of the group (1), i.e. Magnet on Group was treated by PMF within 12 sessions over one month period by the rate of 3 sessions per week in addition to the traditional physical therapy program.
Each patient in the group (2), i.e. magnet off Group was treated by traditional physical therapy program only.
Each patient in the group (3), i.e. vitamin C group was treated by PMF and traditional physical therapy program followed by taking vitamin C (2,000 mg per day).
Each patient in the group (4), i.e. vitamin E group was treated by PMF and traditional physical therapy program followed by taking vitamin E (15 mg per day).
Data Analysis
Data Collection:
Data were collected before and after the study period (1 month) for all groups. The data collection took the same sequence and procedure in patients of all groups. These data included the parameters of CBC and RBCs morphology.
Statistical design:
Statistical analysis was done in order to reach the objectives of the study.
Data collected from this study were statistically analyzed using descriptive statistics using the mean and standard deviation for all groups.
The comparison was made by inferential statistics using MANOVA and paired t-test to determine the probability level for the difference in mean value between the results observed before and after the period of one month in each group.
Results
Data obtained from the four groups regarding RBCs, HB, PCV, and MCV were statistically analyzed and compared.
General characteristics of the subjects:
Overall, Sixty adult subjects were participated in this study. General characteristics of these patients are shown in table (1) and figure (2).
ANOVA test was used to compare the mean age of the four groups’ subjects and revealed that there was no significant difference between the groups (p = 0.159).
|
Table 1: Descriptive statistics and ANOVA test for the mean age of the four groups |
|||||||
|
|
Group I |
Group II |
Group III |
Group IV |
F- value |
p- value |
Sig |
|
X |
X |
X |
X |
||||
|
Age (years) |
30.77 ± 4.38 |
28.33 ± 3.65 |
4.93 |
26.9 ± 5.28 |
2.647 |
0.159 |
NS |
X :Mean, SD: Standard deviation, p value: Probability value, NS: Non significant
:Mean, SD: Standard deviation, p value: Probability value, NS: Non significant
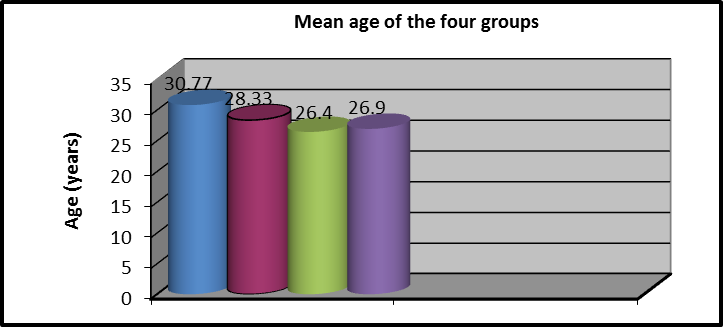 Figure 2: Mean age (years) of groups
Figure 2: Mean age (years) of groups
Effect of the electromagnetic field and vitamins E and C on the morphology of RBCs:
As shown in table 2, 2-way MANOVA was conducted to investigate the effect of EMF and vitamins E and C on RBCs morphology (RBCs, HB, PCV, and MCV). No significant interaction influence of treatment and time (P = 0.891),no considerable effect of therapy (P=0.095), and no considerable effect of time (P= 0.054) were observed.
|
Table 2: two-way MANOVA of the effect of EMF and vitamin C or E on RBCs morphology |
|
|
MANOVA |
|
|
Interaction effect (Time * treatment) |
|
|
F = 0.381 |
P = 0.891 |
|
Effect of treatment (group effect) |
|
|
F = 3.87 |
P = 0.095 |
|
Effect of time |
|
|
F = 3.18 |
P = 0.054 |
|
Table 3: Results of MANOVA between pre and post-treatment mean values of RBCs morphology among groups. |
||||
|
Item |
Pre-treatment Mean±SD |
Post-treatment Mean ±SD |
|
P value |
|
RBCs
|
|
|
|
|
|
Group I |
4.7 ±0.43 |
4.44 ±0.72 |
-5.5% |
0.001 |
|
Group II |
4.6 ±0.35 |
4.69 ±0.84 |
-1.9% - - |
0.739 |
|
Group III |
4.9 ±0.29 |
4.71 ±0.81 |
-3.9%
|
0.001 |
|
Group IV |
4.81 ±0.31 |
4.65±0.80 |
-3.3% |
0.001 |
|
(P-value) |
0.342 |
0.142 |
|
|
|
HB |
|
|
|
|
|
Group I |
13.3 ±1.5 |
12.1 ±1.39 |
-9% |
0.001 |
|
Group II |
13.2 ±1.4 |
13.1 ±1.34 |
-0.7% -0.7%
|
0.334 |
|
Group III |
13.6±1.25 |
12.9 ±1.4 |
-5.1% - |
0.001 |
|
Group IV |
14.1 ±1.19 |
13.4±1.19 |
-5% |
0.001 |
|
(P-value) |
0.252 |
0.094 |
|
|
|
PCV |
|
|
|
|
|
Group I |
40.33 ±3.7 |
38.5 ±3.75 |
-4.5% |
0.001 |
|
Group II |
40.26 ±4.08 |
40.04 ±4.11 |
-0.5%
- |
0.001 |
|
Group III |
41.5 ±3.58 |
40.44 ±3.78 |
-2.5% |
0.021 |
|
Group IV |
43.19 ±3.2 |
42.11±3.17 |
-2.5% |
0.001 |
|
(P-value) (P-value) |
0.109 |
0.08 |
|
|
|
MCV |
|
|
|
|
|
Group I |
86.17 ±8.24 |
87 ±8.5 |
1% |
0.001 |
|
Group II |
85.5 ±4.2 |
85.26 ±4 |
-0.3% |
0.291 |
|
Group III |
84.8 ±5.6 |
85.7 ±5.9 |
-1.1% |
0.013 |
|
Group IV |
89.6 ±3.16 |
90.52 ±3.17 |
1% |
0.001 |
|
(P-value) |
0.101 |
0.064 |
|
|
Comparison of RBCs of the four groups
As shown in table 3 and Figures (3,4), MANOVA test was conducted and revealed that:
There was no statistically significant difference in pre and post-treatment RBCs mean values among the four groups (P>0.05).
In group I, the mean values of pre and post-treatment RBCs were (4.7 ±0.43) and (4.44 ±0.72), respectively. There was a statistically considerable variation (P=0.001).
In group II, the mean values of pre and post-treatment RBCs were (4.6 ±0.35) and (4.69 ±0.84), respectively and no statistically considerable variations were observed (P=0.739).
In group III, the average values of pre and post-treatment RBCs were (4.9 ±0.29) and (4.71 ±0.81), respectively and statistically considerable variations were observed (P=0.001).
In group IV, the mean values of pre and post-treatment RBCs were (4.81 ±0.31) and (4.65±0.80) respectively, and a statistically significant difference was observed (P=0.001).
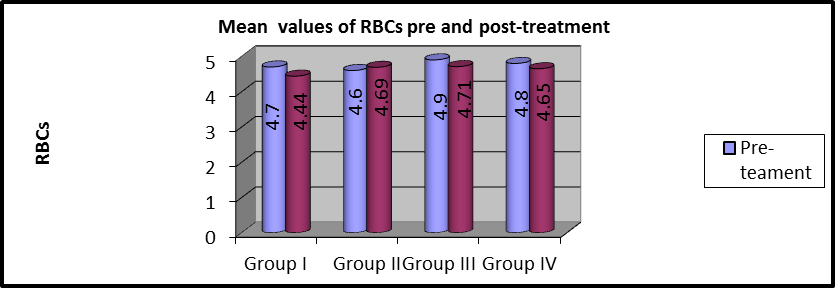
Figure 3: Mean RBCs values of pre and post-treatment within each group.
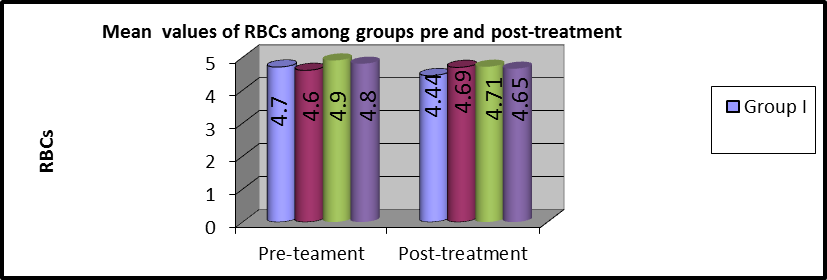 Figure 4: Mean RBCs values of pre and post-treatment among groups
Figure 4: Mean RBCs values of pre and post-treatment among groups
Comparison of Hb of the four groups
As shown in table 3 and Figures (5,6), MANOVA test was conducted and revealed that there was no statistically significant difference between the pre and post-treatment Hb mean values among the four groups (P>0.05).
In group I, the mean values of pre and post-treatment Hb were (13.3 ±1.5) and (12.1 ±1.39), respectively and there were statistically considerable variations (P=0.001).
In group II, the mean values of pre and post-treatment Hb were (13.2 ±1.4) and (13.1 ±1.34), respectively and there were no statistically considerable variations (P=0.334).
In group III, the average values of pre and post-treatment Hb were (13.6 ±1.25) and (12.9 ±1.4), respectively and there were statistically considerable variations (P=0.002).
In group IV, the mean value of pre and post-treatment Hb were (14.1 ±1.19) and (13.4±1.19), respectively, and there was a statistically significant difference (P=0.002).
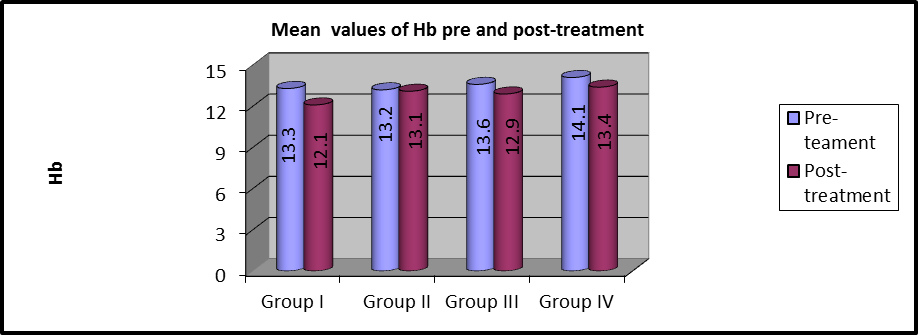 Figure 5: Mean Hb values between pre and post-treatment within each group.
Figure 5: Mean Hb values between pre and post-treatment within each group.
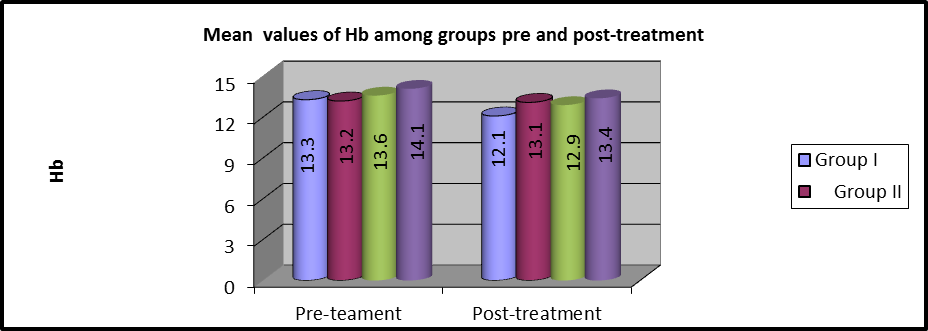 Figure 6:Mean Hb values of pre and post-treatment among groups.
Figure 6:Mean Hb values of pre and post-treatment among groups.
Comparison of PCV between the four groups
As shown in table 3 and Figures (7, 8), MANOVA test was conducted and revealed that there was no statistically significant difference between the pre and post-treatment PCV mean values among the four groups (P>0.05).
In group I, the mean values of pre and post-treatment PCV were (40.33 ±3.7) and (38.5 ±3.75), respectively and there were statistically considerable variations ( (P=0.001).
In group II, the mean values of pre and post-treatment PCV were (40.26 ±4.08) and (40.04 ±4.11), respectively and there were statistically considerable variations (P=0. 001).
In group III, the average values of pre and post-treatment PCV were (41.5 ±3.58) and (40.44 ±3.78), respectively and there were statistically considerable variations (P=0.021).
In group IV, the mean values of pre and post PCV were (43.19 ±3.2) and (42.11±3.17), respectively, and there were statistically considerable variations (P=0.001).
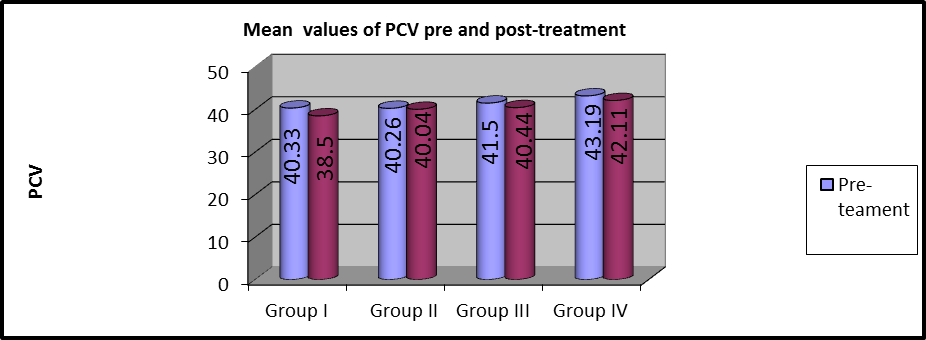 Figure 7: Mean PCV values of pre and post-treatment within each group.
Figure 7: Mean PCV values of pre and post-treatment within each group.
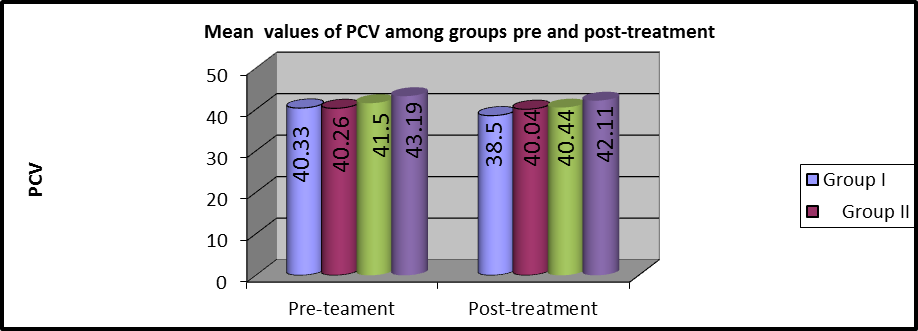 Figure 8:Mean PCV values of pre and post-treatment among the groups
Figure 8:Mean PCV values of pre and post-treatment among the groups
Comparison of MCV between the four groups
As shown in table 3 and Figures (9,10), MANOVA test was conducted and revealed that there was no statistically significant difference in the pre and post-treatment MCV mean values among the four groups (P>0.05).
In group I, the mean values of pre and post-treatment MCV were (86.17 ±8.24) and (87 ±8.5), respectively and there were no statistically considerable variations (P=0.001).
In group II, the mean values of pre and post-treatment MCV were (85.5 ±4.2) and (85.26 ±4), respectively and there were no statistically considerable variations (P=0. 291).
In group III, the mean values of pre and post-treatment MCV were (84.8 ±5.6) and (85.7 ±5.9), respectively and there were statistically considerable variations (P=0.013).
In group IV, the mean values of pre and post-treatment MCV were (89.6 ±3.16) and (90.52 ±3.17), respectively and there was a statistically significant difference (P=0.001).
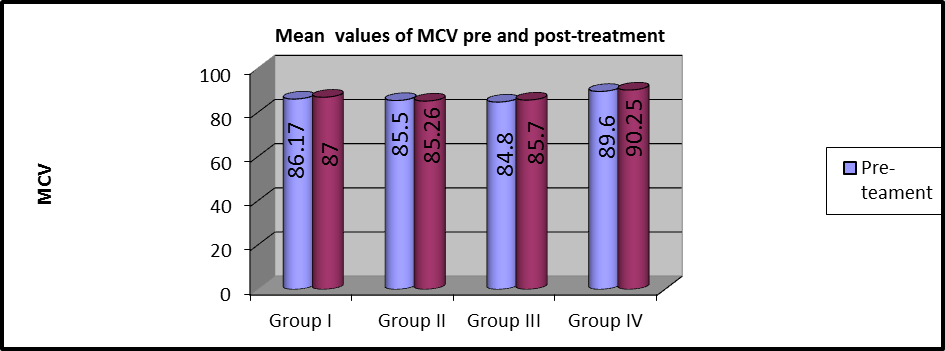 Figure 10: Mean MCV values of pre and post-treatment within each group.
Figure 10: Mean MCV values of pre and post-treatment within each group.
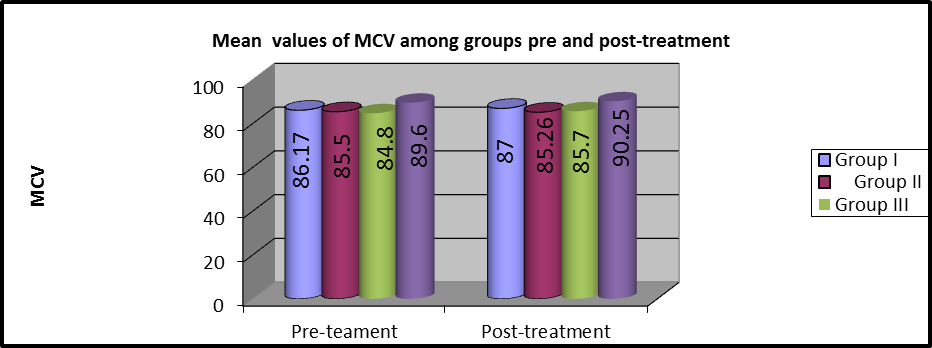 Figure 11: Mean MCV values of pre and post-treatment among groups
Figure 11: Mean MCV values of pre and post-treatment among groups
Discussion
In our study, we noticed a decrease in RBCs, HB, PCV, and MCV compared to the control group. There was a significant improvement of RBC morphology after treatments in the groups by PMF in addition to vitamins E and C.
These results were supported by Cheng et al., 2005 [22] who indicated that the concentration of Hb in living RBCs was lowering and the molecular structure of Hb was varied by the electromagnetic action. The structure of RBCs membrane can be changed. PMF change the shape, structure, and assignment of the cell membrane and affects the structure and role of intracellular material, like the structural variation of intracellular protein and the laceration of the DNA chain. After PMF exposure, the components of the membrane and cytoskeleton can be recombined, the permeation of cell membrane excesses and the structure of RBCs membrane can be varied. In the finding, the Hb and ions progressive from RBCs, and the absorbance of Hb was lowering.
These results come in agreement with the work of Yao et al., 2005 [23] who observed the alteration in the structure of hemoglobin interior the RBCs membrane, influencing the physiological role and capacity of oxygen transport, as shown through a lowering in the hemoglobin concentration after exposure to electromagnetic. Our results are consistent with the study conducted by Forgacs et al., 2005 [24] in which the results showed several hematological variables such as RBCs, hematocrit, hemoglobin, and platelet levels are sensitive to PMF exposure.
These results are in agreement with Rusnani et al., 2008 [25] who observed that there was a decrease in RBC after the fifth month of PMF exposure, which corroborates the findings of this work. In contrast, Zsolt et al., 2006 [26] reported the greatest values in RBC and PCV after exposure of mice with PMF only for the duration of 2 hours/day for 2 weeks on work days.
The effects of PMF on RBCs have been reported by many authors in an attempt to reveal the biological effects associated with PMF exposure. One of the animal studies concluded that exposing the rats to PMF of 50 Hz, resulted in change in the RBCs membrane deformability and solubilization, this remarkable changes may be due to the failure of ATP function in the erythrocytes that might occur under the influence of PMF or the change in the hormonal balance and level due to irradiation, which disturbs the enzymatic activity [27].
Exposure of albino rats to 50 Hz, 0.2 mT PMF resulted in the reduction of RBCs membrane elasticity and permeability and alterations in the molecular structure of hemoglobin. The electrocardiograph (ECG) of the exposed animals was significantly altered. The results also observed that there was no sign of reforming in the newly generated RBCs’ structure after eliminating the animals from the magnetic field, which showed that the blood generating system was dangerously damaged. The disturbance in the heart of the rats was designated to the damage of some physiological roles of the RBCs as a finding of the animals' exposure to the PMF [28].
These results come in agreement with the work of Abdel Aziz et al., 2010 [17] who found that there was a significant decrease in RBC, HB, MCV, and PCV. Moreover, he found that there were signs of improvement in the hematological parameters through therapies with the electromagnetic field along with vitamin C or E.
Amara et al., 2006 and Chater et al., 2006 [29,30] found that regular exposure to PMF can increase the volume of plasma, leading to decrease in the RBC and hemoglobin concentrations in the blood.
Attia et al 2002, Al-Glaib et al., 2008 and Zaghloul, 2011 [31-33] reported that PMF has an adverse effect on hemopoietic tissue, which cause a decrease in RBCs count due to destruction in the spleen tissues. The spleen is a lymphatic organ, which stores blood corpuscles. Any abnormality in the spleen as a result of PMF can lead to hemolysis or destruction of RBCs within the spleen [33]. The spleen hyperfunction increased the destruction rate of RBCs, which could ultimately lead to a decrease in hemoglobin concentration [34].
Many kinds of research showed that no side influences of PMF energizing by characterizing therapy response and determination of blood, serum, liver, and kidney functions. None of them reported other side effects and PMF has a long term record of safety [35,36].
These results are contradicted with the work of In Dasdag et al., 2002 [37] who found that some of the hematologic parameters (RBCs; hemoglobin; hematocrit) were similar in both groups. The results suggest that PMF does not affect the hematologic parameters.
Conclusions
This investigation was conducted to research the influence of PMF with frequency 50 Hz, the intensity of 20 gausses and duration of 20 min on RBCs morphology. In this study, we also researched the therapeutic influence of vitamins E and C on the patients while exposing to PMF.
It was concluded that the morphology of RBCs is influenced by PMF exposure. Furthermore, most of these alterations were showed that the signs of advancements with vitamins E and C therapies than to PMF exposure alone.
Acknowledgments:
The authors express their sincere gratitude to all subjects who kindly participated in the study.
Financial support and sponsorship:
Self-funded.
Conflicts of interest:
NIL.
References
- Delgado JM, Leal J, Monteagudo JL, Gracia MG. Embryological changes induced by weak, extremely low frequency electromagnetic fields. Journal of Anatomy. 1982 May;134(Pt 3):533.
- Hazlewood CF, Markov MS, Kostarakis P. Magnetic fields for relief of myofascial and/or low back pain through trigger points. InProceedings of Forth International Workshop Biological effects of electromagnetic fields 2006 Oct (pp. 475-483).
- Gordon GA. Designed electromagnetic pulsed therapy: clinical applications. Journal of Cellular physiology. 2007 Sep;212(3):579-82.
- Soh YA, Aeppli G. Applied physics: Making sense of magnetic fields. Nature. 2002 May;417(6887):392.
- Al-Rajhi MA. Evaluation of the Risks of Very Low Frequency and Non-Ionizing Electromagnetic Radiations Emitted by Electric Current of 50/60 Hz, its Biological Effects, and Organizations of Legislating Exposure Limits. Science. 2006;18(1).
- Rekecho K. Electricity and Environmental Pollution, the Effect of Electromagnetic Fields on Health. AL-Zad Company, Jeddah. 1999.
- Hassan NS, Abdelkawi SA. Changes in molecular structure of hemoglobin in exposure to 50 Hz magnetic fields. Nature. 2010;8(8):236-43.
- Usman AD, Ahmad WW, Ab Kadir MZ, Mokhtar M, Ariffin R. Effect of radiofrequency electromagnetic field exposure on hematological parameters of mice. World applied sciences journal. 2012;16(5):656-64.
- Calabro, Studies on the bioprotective effectiveness of trehalose of human hemoglobin aqueous solutions under 50 Hz electromagnetic field exposure, Journal of Physical Chemistry B, 2012; 114(37): 12144-1214
- Forgács Z, Kubinyi G, Sinay G, Bakos J, Hudák A, Surján A, Révész C, Thuróczy G. Effects of 1800 MHz GSM-like exposure on the gonadal function and hematological parameters of male mice. Magyar onkologia. 2005;49(2):149-51.
- Strašák L, Vetterl V, Šmarda J. Effects of low-frequency magnetic fields on bacteria Escherichia coli. Bioelectrochemistry. 2002 Jan 1;55(1-2):161-4.
- Yokus B, Cakir DU, Akdag MZ, Sert C, Mete N. Oxidative DNA damage in rats exposed to extremely low frequency electro magnetic fields. Free Radical Research. 2005 Mar 1;39(3):317-23.
- Wolf FI, Torsello A, Tedesco B, Fasanella S, Boninsegna A, D'Ascenzo M, Grassi C, Azzena GB, Cittadini A. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: possible involvement of a redox mechanism. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2005 Mar 22;1743(1-2):120-9.
- Simkó M, Mattsson MO. Extremely low frequency electromagnetic fields as effectors of cellular responses in vitro: possible immune cell activation. Journal of cellular biochemistry. 2004 Sep 1;93(1):83-92.
- Frahm J, Lantow M, Lupke M, Weiss DG, Simkó M. Alteration in cellular functions in mouse macrophages after exposure to 50 Hz magnetic fields. Journal of Cellular Biochemistry. 2006 Sep 1;99(1):168-77.
- Oral B, Guney M, Ozguner F, Karahan N, Mungan T, Comlekci S, Cesur G. Endometrial apoptosis induced by a 900-MHz mobile phone: preventive effects of vitamins E and C. Advances in therapy. 2006 Nov 1;23(6):957-73.
- Aziz IA, El-Khozondar HJ, Shabat M, Elwasife KH, Mohamed-Osman A. Effect of electromagnetic field on body weight and blood indices in albino rats and the therapeutic action of vitamin C or E. Romanian Journal of Biophysics. 2010;20(3):235-44.
- Lewis S.M., Bain B.J., Bates I. Dacie and Lewis Practical Haematology, Churchill Livingstone, USA, 2006.
- Van Nguyen J, Marks R. Pulsed electromagnetic fields for treating osteo-arthritis. Physiotherapy. 2002 Aug 1;88(8):458-70.
- Reed BV, Ashikaga T, Fleming BC, Zimny NJ. Effects of ultrasound and stretch on knee ligament extensibility. Journal of Orthopaedic & Sports Physical Therapy. 2000 Jun;30(6):341-7.
- Shabana A, Mahsen M, Senna M, Steen M. Lumbar disc herrinations: MRI and clinical follow-up in patients treated with traction. The Egyptian Rheumatologist. 2001;23:197-209.
- Cheng Can Yaoi, iaoX Kun Lii and Yao Xiong Huang. Institute of Biomedical Engineering, Jinan University, Guangzhou 510630, Chines Chemical Letters, 2005; 16(8): 1121-1124.
- Yao CC, Li XK, Huang YX. Instant effects of radiofrequency electromagnetic wave on hemoglobin in single living intact red blood cell. Chinese Chemical Letters. 2005 Aug 1;16(8):1121.
- Forgács Z, Somosy Z, Kubinyi G, Bakos J, Hudák A, Surján A, Thuróczy G. Effect of whole-body 1800 MHz GSM-like microwave exposure on testicular steroidogenesis and histology in mice. Reproductive Toxicology. 2006 Jul 1;22(1):111-7.
- Rusnani A, Norhayati MN, Noraini SS, Marina M. Microwave radiation effect—a test on white mice. In2008 IEEE International RF and Microwave Conference 2008 Dec 2 (pp. 262-267). IEEE.
- Zsolt F, Zolt S, Gyorgyi K, Jozsef B, Aranka H, Andras S, Gyorgy T. Effect of whole-body 1800MHz GSM-like microwave exposure on testicular steroidogenesis and histology in mice. Reproductive Toxicology. 2006 Jul;22:111-7.
- Fadel M, Ghanam M, Elrefaei F, Elgebaly R, Abou Elela K, Hueeein A, Surour D, Mohamed I. Effect of non ionizing fields on biological membranes. Twelfth School on Biophysics of Membrane Transport. Poland. 1994 May:62-89.
- Ali FM, S. Mohamed W, Mohamed MR. Effect of 50 Hz, 0.2 mT magnetic fields on RBC properties and heart functions of albino rats. Bioelectromagnetics: Journal of the Bioelectromagnetics Society, The Society for Physical Regulation in Biology and Medicine, The European Bioelectromagnetics Association. 2003 Dec;24(8):535-45.
- Amara S, Abdelmelek H, Salem MB, Abidi R, Sakly M. Effects of static magnetic field exposure on hematological and biochemical parameters in rats. Brazilian Archives of biology and technology. 2006 Nov;49(6):889-95.
- Chater S, Abdelmelek H, Pequignot JM, Sakly M, Rhouma KB. Effects of sub-acute exposure to static magnetic field on hematologic and biochemical parameters in pregnant rats. Electromagnetic biology and medicine. 2006 Jan 1;25(3):135-44.
- Attia AA, Yehia MA. Histological, ultrastructural and immunohistochemical studies of the low frequency electromagnetic field effect on thymus, spleen and liver of albino swiss mice. Pak. J. Biol. Sci. 2002;5(9):931-7.
- Al-Glaib B, Al-Dardfi M, Al-Tuhami A, Elgenaidi A, Dkhil M. A technical report on the effect of electromagnetic radiation from a mobile phone on mice organs. The Libyan journal of medicine. 2008;3(1):8.
- Zaghloul MS. Effects of Chronic Exposure to Static Electromagneti c Field on Certain Histological Aspects of the Spleen and Some Haematological Parameters in Albino Rats. Journal of American Science. 2011;7(8).
- Cabrales LB, Ciria HC, Bruzón RP, Quevedo MS, Céspedes MC, Salas MF. ELF magnetic field effects on some hematological and biochemical parameters of peripheral blood in mice. Electro-and Magnetobiology. 2001 Jan 1;20(2):185-91.
- Huang L, Wang W, Xiao D, Yang L, Lei Z, He C. Effect of pulsed electromagnetic fields of different treatment time on bone mineral density of femur in ovariectomized rats. Zhongguo xiu fu chong jian wai ke za zhi= Zhongguo xiufu chongjian waike zazhi= Chinese journal of reparative and reconstructive surgery. 2008 May;22(5):548-50.
- Ganesan K, Gengadharan AC, Balachandran C, Manohar BM, Puvanakrishnan R. Low frequency pulsed electromagnetic field—a viable alternative therapy for arthritis, 2009.
- Dasdag S, Sert C, Akdag Z, Batun S. Effects of extremely low frequency electromagnetic fields on hematologic and immunologic parameters in welders. Archives of Medical Research. 2002 Jan 1;33(1):29-32.
Contact Meral
Meral Publications
www.meralpublisher.com
Davutpasa / Zeytinburnu 34087
Istanbul
Turkey
Email: [email protected]