|
Original Article |
Sholichah Rohmani1*, Adi Yugatama2, Bambang Bagus Suryadi1, Felicitas Lady F1, Ahmad Ainurofiq3, Fea Prihapsara3
1 Department of Pharmaceutical Technology, Pharmacy D3 Study Program, Faculty of Mathematics and Natural Sciences, Sebelas Maret University Surakarta. 2 Department of Pharmacy Chemical Analysis, A1 Pharmacy Study Program, Faculty of Mathematics and Natural Sciences, Sebelas Maret University Surakarta. 3 Department of Pharmaceutical Technology, Pharmacy Study Program S1, Faculty of Mathematics and Natural Sciences, Sebelas Maret University Surakarta.
Correspondence: Sholichah Rohmani, Department of Pharmaceutical Technology, Pharmacy D3 Study Program, Faculty of Mathematics and Natural Sciences, Sebelas Maret University Surakarta. Email: licha @ staff.uns.ac.id.
|
ABSTRACT Atorvastatin is a statin drug used as the first-line treatment for hyperlipidemia. This patent was used up in 2011. Currently, in Indonesia, there are circulating preparations of atorvastatin tablet innovators, several branded names, and generics. This study was conducted to determine the quality of atorvastatin preparations circulating in Indonesia through physical quality tests in the form of performance tests, weight uniformity, hardness, friability, disintegration time, determination of levels using a UV-Vis spectrophotometer, and dissolution test. Dissolution test using aquades medium and buffer solutions with pH 1.2, 4,5, and 6.8 with a volume of 900 ml, stirring speed of 100 rpm, temperature of 37°C ± 0.5, and testing time for 45 minutes. Tests were carried out on three samples, namely the innovator, branded, and generic atorvastatin tablet samples. All three tablet samples met all physical criteria, including uniformity in weight, hardness, friability, and disintegration time. The results of the determination of atorvastatin levels for innovator, branded, and generic tablets met the content requirements of not less than 90.0%, and no more than 110.0% of the amount stated on the label. The dissolved profile of branded atorvastatin tablets is similar to the innovator, while the profile of generic atorvastatin tablets is not similar to innovator tablets.
Keywords: Atorvastatin, physical quality tests, dissolution profile |
Introduction
Drugs included in the category of biological products, which are ingredients or alloys of materials, used to influence or investigate physiological systems or pathological conditions in the context of establishing the diagnosis, prevention, healing, recovery, and improvement of health, and contraception for humans [1]. By definition, patent medicines or innovator drugs are newly discovered drugs based on research and have a patent period that depends on the type of drug. According to Law No. 14 of 2001, the validity period of patents in Indonesia is 20 years. During those 20 years, the pharmaceutical company has exclusive rights in Indonesia to produce the said drug. Other companies are not permitted to produce and market similar drugs, except if they have a special agreement with the patent owner.
Atorvastatin is one of the drugs whose patent period expired in 2011 [2]. Therefore, we can be sure that there will be a lot of emerging branded or generic names because atorvastatin is one of the statin drugs sold in the market.
Copy drugs, branded named drugs, or branded generic drugs are medicines that have expired patent rights, which are produced and marketed under a trading name. This drug will appear when the originator's patent expires. Atorvastatin is a statin derivative, mainly used to lower blood cholesterol, and for prevention related to cardiovascular disease, which is the major causes of mortalities worldwide [3, 4]. Atorvastatin works by inhibiting HMG-CoA reductase, an enzyme found in liver tissue, which plays a key role in the production of cholesterol in the body [5, 6].
Atorvastatin is the fifth drug in the statin class to be developed; clinical trials show that atorvastatin causes a more dramatic decrease in LDL-C than other statin drugs. From 1996 to 2012, under the trade name of innovator products, atorvastatin became the best-selling drug of all time in the world.
Based on the Regulation of the Head of the Republic of Indonesia Drug and Food Supervisory Agency Number Hk.03.1.23.12.11.10217 of 2011, regarding compulsory drug equivalency testing, atorvastatin is one of the drugs included in the list of copy drugs containing active substances compulsory for bioequivalence testing. The in vitro equivalence test is called the comparable dissolution test, which is a comparative dissolution test conducted to show the similarity of dissolution profiles between the test drug and the innovator/comparator drug. In mandatory copy drugs, the equivalence test is carried out. Equivalence test for copy drugs is done by comparing innovator/comparator drugs [1].
Based on the solubility and permeability, atorvastatin is an insoluble compound with high permeability (Biopharmaceutics Classification System Case-2, BCS II) [7]. Dissolution generally becomes the rate-limiting step in BCS II compounds. Dissolution testing is one of the most important quality controls for pharmaceutical preparations, which can be used to predict bioavailability. Dissolution of a drug is directly related to its pharmacological activity because it is a prerequisite for drug absorption and clinical response. The in-vitro dissolution velocity relationship and its bioavailability are formulated in the form of IVIVC (in-vitro in-vivo correlation) [8].
Based on this description, it is necessary to do further research by comparing the dissolution profile of various atorvastatin tablets circulating in Indonesia, because the atorvastatin tablets in circulation must meet the bioequivalence requirements with the atorvastatin innovator tablets to provide the same therapeutic effect. In addition, the existence of a great deal of research and studies on generic drugs will increase the knowledge of public and health workers.
Materials Dan Method
Tools:
Analytical balance (Sartorius BP 221 S), pH meter, stainless funnel flow meter, stopwatch, hardness tester (Stokes Monsanto), friability tester (Guoming CS-2), disintegration tester, dissolution tool (Electrolab TD-08), vortex, micropipette, UV-Vis spectrophotometer (Hitachi U-2800), cooling cupboards, sonicators, 1 ml injection syringes (Terumo, Philippines), 0.45 µm millipores, and glassware.
Materials:
Standard atorvastatin (Sigma-Aldrich, USA), innovator and generic atorvastatin tablets on the market, phosphoric acid (Merck, Germany), Acetonitrile and Methanol (pro HPLC, Merck, Germany), sterile Aquabidest (PT. Ikapharmindo Putramas, Indonesia) , pH 1.2 buffer solution (hydrochloric acid solution), pH 4.5 buffer solution (acetate buffer solution), and phosphate buffer solution pH 6.8 (phosphate buffer solution).
Research Stages
- Tablet Physical Character Test
- Appearance/Organoleptis
The organoleptic test includes examining the uniformity of color, surface shape, odor, taste, and the presence or absence of physical damage.
- Tablet weight uniformity
A total of 20 tablets, cleaned of dust were weighed one by one, and the average weight was calculated. The percentage value of the deviation and CV were determined in order to obtain variations in the weight of the tablet.
- Tablet hardness
One tablet was placed in the middle and perpendicular to the hardness tester; first in the zero position, then the tool was slowly turned until the tablet broken. The scale achieved was read when the tablet was broken or destroyed [9].
- Friability
A total of 20 tablets, free from dust were weighed, then put into the friability tester. The tool was run for 4 minutes or 100 times/round. The tablets were taken and cleaned of attached particles, weighed again, the percentage of the difference or decrease in weight was calculated. The total weight of the tested tablets should not be less than 1% of the initial weight of the test [9].
- Tablet disintegration time
Six tablets were put in a basket-shaped tube, and then the tubes were regularly raised up-and-down 30 times per minute in a water medium at 36-38 °C. The tablets were stated disintegration if no part of the tablet was left above the screen [10].
- Determination of Atorvastatin Level in Tablet
- Determination of maximum wavelength
100 mg of standard atorvastatin was weighed, dissolved in 100 ml of methanol solution to obtain a concentration of 1 mg/ml, and diluted with methanol to obtain a concentration of 10 µg/ml. The absorbance was read at a wavelength of 200-400 nm [11].
- Making of the atorvastatin calibration curve
Standard atorvastatin concentration was made in methanol with 7 levels (absorbance value on the spectrophotometer in the range 0.2-0.8), each absorbance was read at a maximum wavelength of 246 nm, and calibration curves were made [12].
- Level uniformity
Each atorvastatin tablet was weighed, and then 1 atorvastatin tablet that was weighed earlier, was crushed and dissolved in 25 ml methanol, then homogenized and filtered with filter paper. The dilution was done twice using methanol as a solvent. The first dilution was taken 1.25 ml in 25 ml and then 4 ml in 10 ml. The absorbance was read at the maximum wavelength. The same way was used for other atorvastatin tablets up to 10 tablets. Atorvastatin levels in tablet samples were calculated using a calibration curve [13].
- Tablet Dissolution Test
- Drug release test
rug release test uses a USP type apparatus II dissolution device, with dissolution media buffer solutions with pH 1.2, 4.5, and 6.8, a total volume of 900 ml, and rotation of 50 rpm. Tablets were inserted and 10 ml of the samples were taken at 0, 5, 10, 15, 25, 35, and 45 minutes. Each take 10 ml of dissolution media, was replaced 10 ml with new media so that the media volume remained.
- Drug analysis
Samples were taken and then the levels were analyzed using a UV/Vis spectrophotometer at its maximum wavelength. The maximum wavelength of atorvastatin was previously determined and a standard atorvastatin curve was also made.
Result and Discussion
Preliminary tests conducted were physical tests of atorvastatin tablets, both generic preparations and preparations with trade names, which included appearance, weight diversity, fragility, hardness, and disintegration time. The physical properties of tablets must be checked before a product is marketed.
- Physical Character Test of Atorvastatin Tablet
- Appearance
Tablet manufacturing involves a variety of processes in which some problems may arise in each of the processes involved. In addition to the manufacturing process, there are additional materials or components of the tablet, as well as machines or equipment used in producing tablets, which can affect the quality of the produced tablets [14]. The results of the evaluation of the appearance of generic atorvastatin tablets, innovators, and branded showed no disability or physical damage to the tablets.
- Tablet weight uniformity
FThe parameters used for weight uniformity test were based on Pharmacopoeia of Indonesia (1995). The weight uniformity test was intended to determine the diversity of the availability and to ensure that each tablet contains the same amount and dosage of drugs or active ingredients [10]. Deviations can affect the dose of drug ingredients per tablet. As shown in columns A and B, there was no deviation from the weight in the tablets. Moreover, the results of the CV calculation showed that all atorvastatin tablets on the market, and also generic, innovator, and branded atorvastatin met the weight uniformity requirements. CV values in a row on the generic, innovator, and branded atorvastatin tablets were: 1.01%; 1.53%, and 1.87%, respectively. Of the three types of products, different weights were obtained, which may be caused by the implementation and equipment factors, and each product is produced in a variety of weights that meet the requirements using the standard Good Manufacturing Practices.
|
Table 1. Test Results of Physical Properties of Tablets |
|||
|
Drugs Name |
Column A |
Column B |
CV |
|
Atorvastatin generic |
(387,60 - 428,40) mg |
(367,2 - 448,8) mg |
1,01% |
|
Atorvastatin innovator |
(283,97 - 330,02) mg |
(260,95 - 353,05) mg |
1,53% |
|
Atorvastatin branded |
(280,74 - 326,26) mg |
(257,97 - 349,03) mg |
1,87% |
- Tablet hardness
Tablet preparations should have a certain hardness in order to be able to withstand a variety of mechanical shocks during manufacturing, packaging, and transportation. A good tablet hardness ranges between 4-8 kg [14]. The results showed that the hardness of tablets that met the criteria was atorvastatin generic with a hardness of 7.36 kg, whereas for the atorvastatin innovator tablet with a hardness of 11.24 kg, and atorvastatin branded tablets with a hardness of 11.11 kg, it could be said that they had a high level of violence because of a level of violence above 8 kg. The difference in the hardness between generic products, innovators, and branded products, may also be due to the differences in granulation methods, binders, and lubricants used in the tablet manufacturing process by each manufacturer.
|
Table 2. Tablet Hardness Test Results |
|
|
Drugs Name |
Hardness (kg) |
|
Atorvastatin generic |
7,36 |
|
Atorvastatin innovator |
11,24 |
|
Atorvastatin branded |
11,11 |
- Tablet friability
Tablet hardness is not an absolute indicator of its strength. Therefore, another way to measure the strength of tablets is the fragility index that is often determined. From the results of the study, it was found that the fragility index for the three atorvastatin tablets on the market fulfills the requirements because the result was 0%, although the weight loss was smaller than 0.5% to 1%, it can still be justified. The resistance of tablets to mechanical shocks in the production environment is related to a large number of tablets available, the production equipment used, and the skills of production employees [15].
|
Table 3. Tablet Friability Test Results |
|
|
Drugs Name |
Friability Index (%) |
|
Atorvastatin generic |
0 |
|
Atorvastatin innovator |
0 |
|
Atorvastatin branded |
0 |
- Tablet disintegration time
According to USP (2012) for film-coated tablets, each test tablet should completely be destroyed within a maximum of 30 minutes. [16] Of the three types of tablets studied, all of them met the requirement because the destruction time was less than 30 minutes. The longest disintegration time was for atorvastatin generic tablets, and the fastest disintegration time was for the innovator atorvastatin tablet. The results of the crushed time test were inversely proportional to the results of the hardness test, where the highest hardness was for the innovator atorvastatin tablet and the lowest hardness was for generic atorvastatin tablet. Hard tablets tend to take longer to break or disintegrate. This may be influenced by the formulation factors of each producer, density, and the amount of the prepared ingredients, including all added auxiliaries especially the binder (granulation material), as well as the lubricant, size, and shape.
|
Table 4. Disintegration Time Test Result of Tablets |
|
|
Drugs Name |
Disintegration Time |
|
Atorvastatin generic |
13 minute 41 seconds |
|
Atorvastatin innovator |
1 minute 25 seconds |
|
Atorvastatin branded |
1 minute 44 seconds |
- Determination of Atorvastatin Tablet Levels
- Determination of Maximum Wavelength
In determining the maximum wavelength, it aims to find out at what absorption the substance can be read by the UV spectrophotometer optimally. The maximum wavelength is determined by scanning wavelengths between 400-200 nm. This range was chosen because the analyte was colorless, so scanning was done in the UV region. The peak used is the peak at the absorption wavelength of 246.2 nm. Based on the research of Sawant, et al. (2012), the maximum wavelength of atorvastatin is 246 nm. The wavelength difference of 0.2 nm is still within the allowable tolerance range according to the Ministry of Health of the Republic of Indonesia (1995), which is approximately 3 nm.
Figure 1. Maximum Wavelength Spectrum
- Atorvastatin Calibration Curve
Methanol was used as a solvent in making the atorvastatin calibration curve. The absorbance obtained was measured at the maximum wavelength and then a linear regression equation was made to determine the value of the linear correlation. The linearity parameter used in making the calibration curve is the coefficient of determination (R2). From the atorvastatin calibration curve in methanol, the correlation value r = 0.9998, and the coefficient of determination (R2) which is close to 1 is 0.9996. Linearity is recognized if the coefficient of determination (R2) is more than 0.997 [17]. Based on the value of r, the equation y = 0.0339x + 6.704 x 10-5 can be obtained; y is absorbance, and x is the concentration (µg/ml). The graph shows a linear relationship between concentration and absorbance. In this research, a validation test was conducted with the limit of detection (LOD) method and the limit of quantitation (LOQ). LOD is the value of the substance concentration measured when the instrument starts detecting the presence of substances, while LOQ is the lowest concentration value of the substance measured when the instrument starts detecting the substance with good accuracy and precision. LOD and LOQ values can be determined from the value of the signal to noise (S/N). LOD value is the concentration value when S/N = 3, while the LOQ value is the concentration value when S/N = 10 [18]. The LOD and LOQ values obtained from this research were 0.3420 mg/L and 1.1402 mg/L, respectively. Thus, in making the concentration series for the calibration curve, the smallest concentration used should be above the LOQ value. The smallest concentration used in this research was 5 mg/L, which was already above the LOD and LOQ values.
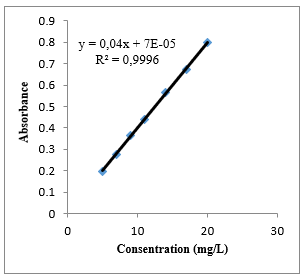
Figure 2. Atorvastatin Calibration Curve
- Determination of Tablet Content
he results of the determination of atorvastatin drug levels for the innovator, branded, and generic tablets met the content requirements, which were not less than 90.0%, or more than 110.0% of the amount stated on the label [19].
|
Table 5. Test Results of Atorvastatin Tablets |
|||
|
|
Drug Level (%) |
||
|
Tablet |
Atorvastatin Generic |
Atorvastatin Innovator |
Atorvastatin Branded |
|
1 |
105,962 |
102,344 |
105,493 |
|
2 |
104,356 |
98,981 |
99,621 |
|
3 |
104,741 |
106,209 |
98,152 |
|
4 |
105,362 |
101,198 |
101,198 |
|
5 |
102,399 |
104,865 |
97,652 |
|
6 |
101,49 |
98,333 |
98,782 |
|
7 |
104,985 |
101,351 |
98,433 |
|
8 |
99,808 |
102,2 |
101,132 |
|
9 |
104,486 |
102,459 |
101,83 |
|
10 |
102,946 |
104,515 |
100,939 |
|
SD |
1,941 |
2,486 |
2,335 |
|
RSD (%) |
1,873 |
2,431 |
2,327 |
- Tablet Dissolution Test
- Raw Curve of Atorvastatin Tablet
In making standard curves, the absorbance was measured at the maximum wavelength, then a linear regression equation was made to determine the value of a linear correlation, and these results indicated a linear relationship between concentration and absorbance.
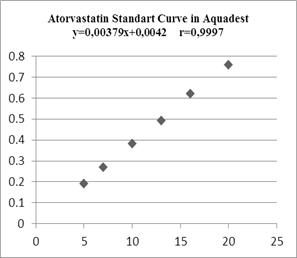
Figure 3. Atorvastatin Standard Curve in Aquades
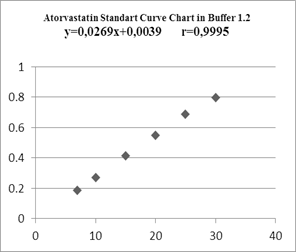
Figure 4. Atorvastatin Standard Curve in Buffer 1,2
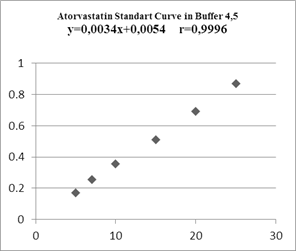
Figure 5. Atorvastatin Standart Curve in Buffer 4,5
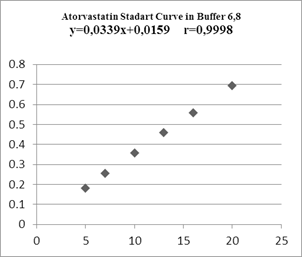
Figure 6. Atorvastatin Standart Curve in Buffer 6,8
- Dissolution Test
Dissolution test is the process of dissolving active substances (medicinal substances) in a drug preparation into a medium. The tablets will undergo a process of disintegration when the drug contacts with body fluids, namely the destruction of the tablet into a granule, and continued with the destruction of the aggregate into its constituent particles. Dissolution testing has several objectives, namely for optimization of formulas, and routine control after fabrication [14]. The dissolution test results of the three tablet samples were plotted in a graphical form and can be seen in the figure 7,8,9,10. In general, the active ingredient in film-coated tablets has dissolved more than 70% in the 15th minute[19]. Atorvastatin branded and innovator tablets have met these requirements, but generic tablets only reach 70% in the 45th minute. None of the pH 1.2 buffer media was dissolved at 70% in the 15th minute, and only samples of branded atorvastatin tablets reached 70% in the 45th minute. In the buffer medium with pH 4.5 in the 15th minute, the release levels of atorvastatin branded tablets and innovator tablets had reached 70%, whereas for generic tablets it did not reach 70%, even though it was at the end of testing in the 45th minute. On buffer media with pH 6.8, the levels of sample of branded atorvastatin tablets and innovator tablets reached 70%, which were released in the 15th minute; while for generic tablets, 70% was released in the 25th minute. There are many factors that cause differences in dissolution profiles between innovator, branded, and generic tablets, including formulations, methods for making tablets, the amount, and the types of excipients used. The preferred oral loose tablets are usually hard but quickly broken ones so that the tablets have resistance during the production, packaging, and distribution processes, but are quickly destroyed so that the active substance can be released immediately, and does not delay the time needed for the drug to affect. Excipients that affect the release of active substances include binders, crushers, and lubricants. Binder is needed because tablets are expected to meet friability requirements, which aim to be undamaged when the drug is distributed. Meanwhile, the destructive agent seeks to quickly make the drug disintegrate, usually through a liquid absorption mechanism, which makes the drug swell, then disintegrate. In addition to binders and crushers, lubricants are other excipients that can affect dissolution. Too much sliding, which is usually hydrophobic, will prevent the destruction of tablets and the release of active substances [19].
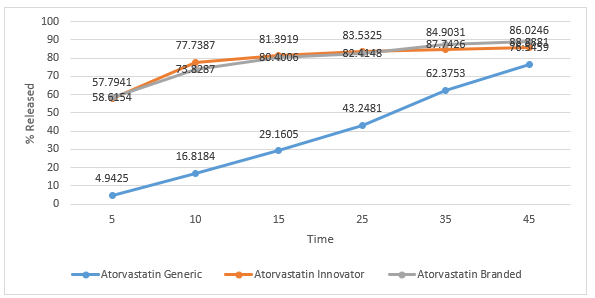
Figure 7. Graph of Dissolution Profile of Atorvastatin Tablets in Medium Aquades
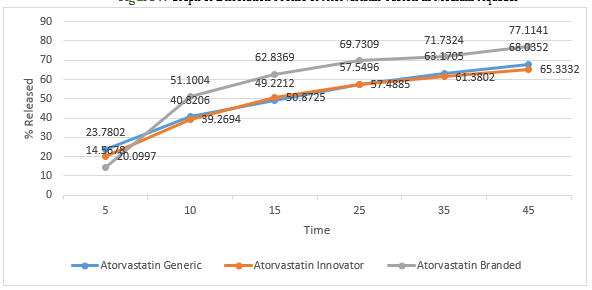
Figure 8. Graph of Dissolution Profile of Atorvastatin Tablets in Medium Buffer pH 1,2
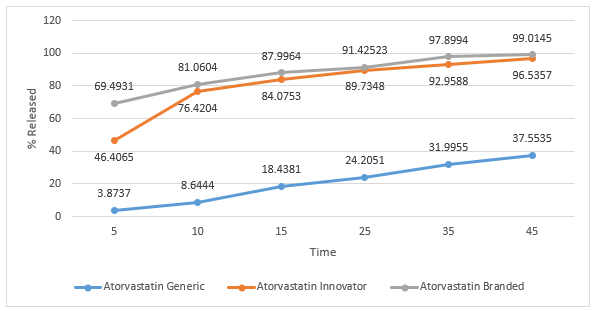
Figure 9. Graph of Dissolution Profile of Atorvastatin Tablets in Medium Buffer pH 4,5
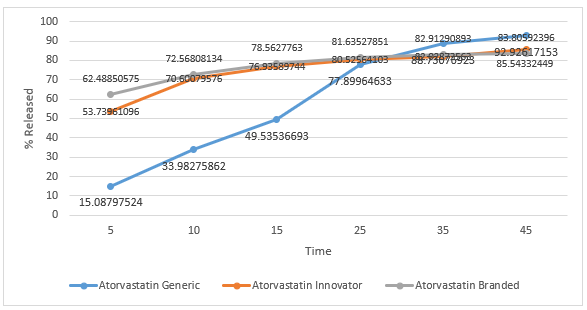
Figure 10. Graph of Dissolution Profile of Atorvastatin Tablets in Medium Buffer pH 6,8
- Results of Release Kinetics Analysis
The kinetics of drug release from each test product is known by making a curve between the cumulative amount of atorvastatin release and time. Furthermore, the results of drug release are related to the zero-order, first-order, Higuchi, and Korsmeyyer-Peppas equations. The above results show that in the aquades medium, all the generic, innovator, and branded atorvastatin tablet samples followed the release of Korsika Korsmeyer Peppas. On the medium with pH 1.2, generic and branded tablets followed the Higuchi kinetics, while the innovator tablets followed the Korsmeyer Peppas kinetics. On the buffer media with pH 4.5 and 6.8, generic tablets followed the Higuchi kinetics, while the other tablets followed the Korsmeyer Peppas kinetics.
|
Table 6. Results of Atorvastatin Release Kinetics Analysis According to Zero Zero-Order Kinetics |
|||||
|
Type of Atorvastatin Tablets |
Parameter |
Aquades |
Buffer Solution pH 1,2 |
Buffer Solution PpH 4,5 |
Buffer Solution PpH 6,8 |
|
Generic |
R2 |
0,9925 |
0,9118 |
0,9651 |
0,9118 |
|
Innovator |
R2 |
0,6719 |
0,7906 |
0,6719 |
0,7138 |
|
Branded |
R2 |
0,8473 |
0,6602 |
0,8473 |
0,7220 |
|
Table 7. Results of Atorvastatin Release Kinetics Analysis According to First First-Order Kinetics |
|||||
|
Type of Atorvastatin Tablets |
PParameter |
Aquades |
Buffer Solution pH 1,2 |
Buffer Solution pH 4,5 |
Buffer Solution pH 6,8 |
|
Generic |
R2 |
0,8107 |
0,7510 |
0,8092 |
0,7808 |
|
Innovator |
R2 |
0,5255 |
0,6649 |
0,5900 |
0,6601 |
|
Branded |
R2 |
0,7058 |
0,5069 |
0,8105 |
0,6923 |
|
Table 8. Results of Atorvastatin Release Kinetics Analysis According to Higuchi Kinetics |
|||||
|
Type of Atorvastatin Tablets |
Parameter |
Aquades |
Buffer Solution PpH 1,2 |
Buffer Solution PpH 4,5 |
Buffer Solution pH 6,8 |
|
Generic |
R2 |
0,9934 |
0,9432 |
0,9883 |
0,9690 |
|
Innovator |
R2 |
0,6818 |
0,8871 |
0,7829 |
0,8137 |
|
Branded |
R2 |
0,8537 |
0,7739 |
0,9273 |
0,8332 |
|
Table 9. Results of Kinetics Analysis of Atorvastatin Tablet Release According to Korsmeyer Peppas Kinetics |
|||||
|
Type of Atorvastatin Tablets |
Parameter |
Aquades |
Buffer Solution pH 1,2 |
Buffer Solution pH 4,5 |
Buffer Solution PpH 6,8 |
|
Generic |
R2 |
1 |
0,9422 |
0,9671 |
0,9623 |
|
Innovator |
R2 |
0,7758 |
0,8907 |
0,8286 |
0,8818 |
|
Branded |
R2 |
0,9101 |
0,7602 |
0,9684 |
0,9123 |
Conclusion
Generic, innovator, and branded atorvastatin tablet samples met all the physical properties requirements of the tablets and met the levels of active substances in the tablets. The dissolved profile of branded atorvastatin tablets was similar to the innovator, while the profile of generic atorvastatin tablets was not similar to the innovator tablets.
Acknowledgment
The authors thank the Universitas Sebelas Maret Surakarta for funding this research through an MRG grant.
Conflict of interest statement:
The authors declared no conflict of interest.
References
- BPOM. Regulation of the Head of the Republic of Indonesia Drug and Food Supervisory Agency About Mandatory Equivalence Tests. Jakarta: Badan Pengawas Obat dan Makanan, 201
- Moon, J. Switching Statins. s.l.:BMJ, 2006.
- Alghamdi, E.S. Protective Effect Of Quinoa (Chenopodium Quinoa Willd.) Seeds Against Hypercholesterolemia In Male Rats. Pharmacophore, 2018; 9(6): 11-21 .
- Ghorbani, A., Shirzadpour, E., Kaffashian, M. R., Mohamadpour, S., Seifinejad, Y., Amraei, M.. Anti-Atherosclerotic Effects of the Hydroalcoholic Extract of Crocus sativus L. (saffron) Petals on Hypercholesterolemic Rats. International Journal of Pharmaceutical and Phytopharmacological Research, 2018; 8(6): 99-10
- Clearinghouse, N. G. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and. 2012. [Online] Available at: www.guideline.gov/content.aspx?1d=37693 [Accessed 6 6 2017].
- Alshubaily, F.A., Jambi, E.J. The Possible Protective Effect of Sage (Salvia Officinalis L.) Water Extract Against Testes and Heart Tissue Damages of Hypercholesterolemic Rats. International Journal of Pharmaceutical and Phytopharmacological Research, 2018; 8(1): 62-68.
- Benet, L. Z. The Role of BCS (Biopharmaceutics Classification System) and BDDCS (Biopharmaceutics Drug Disposition Classification System) in Drug Development. J Pharm Sci, 2013; 102(1): 34-42.
- Cardot, J. M., Beyssac, E., Alric, M. In Vitro-In Vivo Correlation: Importance of Dissolution in IVIVC. Dissolution Technologies, 2007; 15-19.
- Goeswin, A. Pengembangan Sediaan Farmasi. Bandung: ITB, 2006.
- Health, D. O. Pharmacopeia of Indonesia. IV ed. Jakarta: Ministry of Health of the Republic of Indonesia, 1995.
- Kane, P., Desai, D. Simultaneous Spectrophotometric Estimation of Atorvastatin and Fenofibrate in Bulk Drug and Dosage Form by Using Dual Wavelength Method. International Journal of Research in Pharmaceutical and Biomedical Sciences, 2012; 3(4): 1448-1453.
- Sawant, R., Ahmed, R., Ramdin, S. Darade, S. Spectrophotometric Methods For Simultaneous Estimation Of Atorvastatin And Niacin In Tablet Dosage Form. International Research Journal of Pharmacy, 2012; 3(5): 364-367.
- Devi, R., Ramakrishna. New Spectrophotometric Methods For Simultaneous Determination Of Amlodipin Besylate And Atorvastatin Calcium In Tablet Dosage Form,. International Reseach Journal of Pharmacy and Pharmaceutical Sciences, 2010; 2(4): 1322-1331.
- Hadisoewignyo, L., Fudholi, A. Solid Dosage Form. Yogyakarta: Pustaka Pelajar, 2013.
- Lachman, L., Lieberman, H., Kanig, J. The Theory and Practice of Industrial Pharmacy. 3 ed. Philadelphia: Lea&Febiger, 1986.
- USP, The United States Pharmacopeial. 32 ed. USA: The United States Pharmacopeial, 2012.
- Chan, C. C., Lam, H., Lee, C., Zhang, X. Analytical Method Validation and Instrument Performance Verification. s.l.:John Wiley & Sons Inc, 2014.
- Shrivastava, A., Gupta, V. B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. In: M. Pradesh, ed. India: Departement of Pharmaceutical Analysis, 2011; 21-25.
- Aini, N., Saraswati, R. D. Sari, I. Profil Disolusi Terbanding, Penetapan Kadar, dan Kualitas Fisik Tablet Atorvastatin Inovator, Generik Bernama Dagang dan Generik. Jurnal Kefarmasian Indonesia, 2015; 5(2): 90-97.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
