Effect of TDCS vs. task-specific training on Spatiotemporal gait
Samah M. Abdelazeim Elsadany1*, Faten H. Abdelazeim2, Mohamed Elsayed Elawady3
1Pediatric Specialized Hospital, Cairo University, Egypt. 2 Pediatric Physical Therapy Department, Faculty of Physical Therapy, Cairo University, Egypt. 3Neurological Department, Faculty of Medicine, Cairo University, Egypt.
Correspondence: Samah M. Abdelazeim Elsadany, PhDResearcher, Specialized Physiotherapist- Pediatric Specialized Hospital, Cairo University. Email: dr.samah_mohamed @ yahoo.com
|
ABSTRACT
Background and Objective: Diplegic cerebral palsy children severed from primary functional problem expressed as abnormal gait pattern leading to limitation of mobility and activity of daily living. This study was aimed to compare the long-lasting effect of transcranial direct current stimulation and task-specific training in the form of treadmill on spatiotemporal gait parameter in children with diplegic cerebral palsy. Method: A randomized –trial was conducted involving 40 children with spastic diplegic cerebral palsy between six to ten years of age. The spatiotemporal gait parameters were evaluated for all children 3 times, 1st evaluation (pre-treatment), 2nd evaluation by the end of 2nd week after the studied interventions (post-treatment 1) and 3rd evaluation by the end of 12 successive weeks after the treatment program completion (post-treatment 2). The participants were assigned randomly into two groups (A & B), both groups were received selected physical therapy gait training program for 5 times/week for two successive weeks in addition to selected intervention, then 3 sessions/week for the remaining of treatment program for 10 weeks, group (A) was submitted to active transcranial direct current stimulation and group (B) was submitted to treadmill training and sham transcranial direct current stimulation, each intervention was applied for 5 times/week for two successive weeks (total of 10 sessions). Result: there was a significant improvement in both groups regarding selected spatiotemporal gait parameters (step length and gait velocity) with better results in tDCs group compared to the treadmill group; furthermore tDCs group showed an increase in spatiotemporal gait parameters for 10 successive weeks following the intervention completion. Conclusion: tDCS promoted a great improvement and long-lasting effect on spatiotemporal gait parameters in children with diplegic cerebral palsy. Keywords:Transcranial direct current stimulation, treadmill, spatiotemporal gait, diplegia, cerebral palsy. |
Introduction
Diplegic cerebral palsy (CP) represents one of the most prevalent lifelong developmental disabilities, that was described as neurophysiological impairment caused by global alterations in subcortical activity with a reduction in the activity of corticospinal and somatosensory circuits [1, 2]. Such children have postural problems due to many reasons, spasticity, excessive muscle weakness can also affect motor development in these children, leading to difficulties in performing basic functional actions including standing, walking, and sitting altering gait pattern [3, 4].
The impairment of gait is present in 90 % of children with diplegic CP due to diminished cortex excitability, lower limbs spasticity, excessive muscle weakness, altered joint kinematics, diminished postural reactions that lead to the lack of coordination and balance. Moreover, inadequate postural control limits motor development in these children [5, 6].
The important goal in children’s rehabilitation with diplegic CP is to facilitate walking and proper gait pattern without or with assistance, which allows such children greater participation in activities for successful daily interactions with family and society as well as better physical development [7, 8].
Thus, treadmill training offers a new therapy environment for these children, which can enhance their motivation to optimize the posture of standing and also gait speed and endurance, the motor treadmill training favor proprioceptive feedback, leading to adjustment for adequate postural balance during the functional performanceand gait [9].
The transcranial is a direct current stimulation, considering as an alternative therapy applied for children with diplegic cerebral palsy to modulate the cerebral cortex activity, potentiate neuroplastic changes, activate the specific neural network, and open a path for the prolongation and enhancement of the functional gains provided by physical therapy [10]. The short-term tDCs application has short-lasting effects, whereas long-term application generates long-lasting effects and more prolonged modulating effect on the cerebral cortex related to plastic mechanisms [11].
This study was aimed to compare the effect of transcranial direct current stimulation TDCs versus treadmill and investigate the long-lasting effect of TDCs and treadmill on the selected spatiotemporal gait parameter in children with diplegic cerebral palsy.
Subject, Materials, and Methods:
The present randomized study (figure 1) received approval from the Human Research Ethical Committee of the Faculty of Physical Therapy, Cairo University under process number (00534/2014). All parents signed a statement of informed consent agreeing to the participation of their children in this study.
Figure 1: Flowchart of study based on consolidated standards of the reporting trials (CONSORT).
Study sample: This study took place at the Armed Forces Center of Physical Medicine and Rehabilitation, Faculty of Physical Therapy and Pediatric Specialized Hospital; Cairo University, from May 2016 to January 2018. Forty children with spastic diplegic cerebral palsy were recruited from the outpatient clinic, Faculty of Physical Therapy and Pediatric Specialized Hospital, Cairo University. The randomization was generated by using a block of six and four to minimize the risk of imbalance size of separated groups. The participants were selected on the basis of the eligibility criteria and were allocated to two groups (A&B). Inclusive Criteria: age ranged from six to ten years, diagnosis of spastic diplegic cerebral palsy; spasticity ranged from 1-1+ grades in Modified Ashworth Scale; classification on Level I, II of Gross Motor Function Classification System (GMFCS); independent ambulation with or without minimal assistance; and the degree of understanding compatible with the proposed procedures. The following were exclusive criteria; the history of orthopedic surgeries or injection with botulinum toxin for the last 2 years; visual or auditory defects; epilepsy or seizures; other neurological disorders; fixed orthopedic deformity in both lower limbs and upper limbs; metal implants in skull or hearing aids.
Evaluation producers
All children of both groups were assessed 3 times, 1st evaluation; before starting the treatment programs (pre-treatment), 2nd evaluation; by the end of 2nd week after the studied intervention (post-treatment 1) and 3rd evaluation; 10 successive weeks after the end of the studied intervention (post-treatment 2). The selected spatiotemporal gait parameters, step length, and velocity were evaluated by using Walkway Teckscan Pressure Assessment System, which provides both tactical and visual feedback in diplegicpatients with neurologic gait dysfunctions. The child started in standing position on the MatScan with head up, arms alongside, barefoot and heels aligned with an unrestricted footbase. The child was instructed to walk on the walkway sensor as his/her normal gait for about 20 steps and the proper child’s foot was selected before the recording started, also it was important, that the child was asked to look forward in front of him/her, with fixation his vision on the wall, and walk toward it.
Intervention
Both groups were received selected physical therapy gait training program; this program was applied for one hour per each session, 5 times/week for 2 successive weeks, then 3 times/week for 10 weeksincluding: walking in close environment in which the child walked (Forward, backward and sideways) dependently through using separator and stepper, walking in an open environment by placing obstacles across walking track as rolls or wedges of different sizes and heights, walking training on different floor surfaces (spongy and hard surface and on a mat), and climbing stairs up and down without support. Training of gait was complicated by a placed stepper on a balance board which needed more concentrations (motor control) from the child to control his walking, and finally facilitation of dynamic balance during walking as an indicator for proper gait pattern in the form of forwarding and sideways walking on balance board and gravity force system. The physiotherapist stood near the children to ensure safety and prevent potential falls.
In addition to previously selected physical therapy, the gait training program, group (A) received active transcranial direct current stimulation. This stimulation was conducted with an intensity of 1 mA for 20 mints per session, 5 times/week for two successive weeks (total of 10 sessions). The active transcranial direct current stimulation was applied with the tDCS device (Active dose II, Active Tek Inc., USA), by using two non-metallic sponge rectangular electrodes measuring 5 ×5 cm2 and moistening with saline solution. According to Kaski et al. [12], the Anodal electrode (+), targeting right and left lower limb motor cortices simultaneously, following the Internationally Standardized 10/20 Electroencephalogram System, was positioned over Cz corresponding to the motor area of the lower limbs, and Cathode electrode (-) was positioned over the inion as shown in (figure 2).
During tDCs session, the child remained seated in a comfortable position, at the beginning of the session for ten seconds; stimulation was gradually increased until reaching 1 mA and gradually reduced in the last ten seconds of the session. The important point that the location of the treatment area was identified accurately under supervision of neurologist according to the 10/20 system of a motor brain map.

Figure 2: Electrodes position of tDCS
Study Group (B) received treadmill training and sham transcranial direct current stimulation, in addition to the previously selected physical therapy gait training program. Treadmill training was performed in 30-minute sessions, 5 times/week for 2 weeks (total of 10 sessions). The velocity was gradually increased based on the child’s tolerance. During the sessions, treadmill speed was maintained at 60 to 80% of the maximum speed, the child walked at 60% maximum speed in the 1st and last 5 min and 80% in the middle 20 minutes according to Grecco et al. [9], treadmill training was performed on a treadmill (Enraf, 2812 wolle, o2-326, Holland). For sham transcranial direct current stimulation, the electrodes were positioned in the same manner as study group (A) and the stimulator was switched on for 30 seconds only, then the children did not receive any electrical stimulation during the rest of their session [13].
Statistical analysis
In this study, the data were tabulated and processed using SPSS v.23, the mean ± standard deviation was demonstrated for all subjects. Student "t" test was used to compare the results of post treatments between both groups at post-treatment 1 and post-treatment 2, considering select spatiotemporal gait parameters, step length, and gait velocity. Paired "t" test was used to compare the results within each group; between pre-treatment & post-treatment 1, between pre-treatment & post-treatment 2, and between post-treatment 1 & post-treatment 2. The level of significance was set at p< 0.05.
Results:
Forty children with diplegic CP were randomly assigned into two groups (A&B). There were not any statistically significant differences between both groups regarding demographic data (age, weight, and height) as shown in table (1).
|
Table 1: Comparison of age, weight, and height between groups |
|||||
|
Age (yr) |
7.95 ± 0.94 |
7.50 ± 1.19 |
1.323 |
0.194 |
Non |
|
Weight (Kg) |
21.80 ± 4.14 |
20.90 ± 3.11 |
0.776 |
0.442 |
Non |
|
Height (cm) |
120 .05 ± 6.40 |
117.90 ± 7.44 |
0.979 |
0.334 |
Non |
P: probability, t: Student test, Non-significant: P < 0.05.
The outcomes of this study, step length and velocity were estimated for 3 times. Velocity: As presented in table (2) and figure (3) considering the "pre" test comparison between both groups did not show any significant differences indicating the homogeneity of groups ,within group (A) there was a significant increase in the velocity in pre to post 1, pre to post 2 and post 1 to post 2 intervention comparisons, while in group (B) there was a significant increase of velocity in pre to post 1 and pre to post 2 treatment comparison, no significant difference in post 1 to post 2 treatment comparison. In the between-group comparison, there were significant differences in the "post 1" test and "post 2" test between both groups, this significant increase in favor of group (A).
|
Table2: Mean ±SD and p values of velocity in pre, post1 and post2 tests at both groups. |
||||||
|
Velocity |
Pre test |
Post 1test |
Post 2 test |
Pre-post1 t-value/ p- value |
Pre-post 2 t-value/ p- value |
Post1-post 2 t-value/ p- value |
|
Mean± SD |
Mean± SD |
Mean± SD |
|
|
||
|
Group A |
33.40±6.60 |
50.16±13.32 |
58.67±11.34 |
-6.308/ 0.000 |
-10.821/ 0.000 |
-2.452/ 0.02 |
|
Group B |
32.58±9.06 |
38.16 ±7.00 |
36.65±8.29 |
-3.822/ 0.001 |
-2.200/ 0.040 |
41.204/ 0.244 |
|
t-value |
0.329 |
3.567 |
7.011 |
|
|
|
|
p- value |
0.744 |
0.001 |
0.000 |
|
|
|
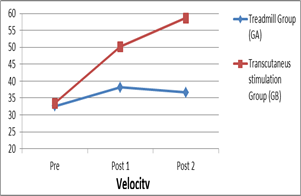
Figure 3: velocity in pre, post 1 and post 2 treatments in treadmill and tDCS group
Step length:
As presented in table (3) and figure (4) considering the "pre" test comparison between both groups did not show any significant differences, indicating the homogeneity of groups. Within group (A) there was a significant increase in pre to post1, pre to post 2, and post 1 to post 2 intervention comparisons, while in the group (B) there was a significant increase in pre to post 1 and pre to post 2 intervention comparisons. No significant difference was observed in post 1 to post 2 intervention comparisons. In the between-group comparison, there were significant differences in "post 1" and post 2" tests between both groups, this significant increase was in favor of group (A).
|
Table 3: Mean ±SD and p values of step length in pre, post1 and post2 tests at both groups. |
||||||
|
Step length |
Pre test |
Post1 test |
Post2 test |
Pre-post1 t-value/ p- value |
Pre-post2 t-value/ p- value |
Post1-post2 t-value/ p- value |
|
Mean± SD |
Mean± SD |
Mean± SD |
|
|
||
|
Group A |
30.300±4.716 |
42.445±6.847 |
48.507±7.251 |
-7.348/ 0.000 |
-10.960/ 0.000 |
-3.531/ 0.002 |
|
Group B |
32.382±5.038 |
37.055±5.005 |
35.115±5.754 |
-3.296/ 0.004 |
-3.156/ 0.005 |
1.764/ 0.094 |
|
t-value |
-1.349 |
2.842 |
6.470 |
|
|
|
|
p- value |
0.185 |
0.007 |
0.000 |
|
|
|
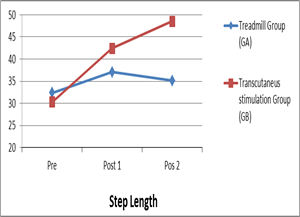
Figure 4: step length in pre, post 1 and post 2 treatments in treadmill and tDCS group
Discussion
Numbers of neuromotor intervention techniques have been applied for improving outcomes in children undergoing motor rehabilitation, which are safely administrating to diplegic CP children. The forms of physical therapy training are no longer sufficient so that either central or peripheral stimulations appear to be away for cortex activation, enhancing motor learning of proper gait, and postural pattern with extending this functional gain achieved by physical therapy [10, 11, 14].
In the present study, both tDCS group and treadmill group demonstrated positive improvement in selected spatiotemporal gait parameters, step length and gait velocity in post-treatment comparison following the different interventions, however, statistically significant differences between the groups were found, with better results in the tDCS group regarding the treadmill group.
In concordance with the study done by Duarte et al.[15] who revealed that gait training combined with anodal transcranial direct-current stimulation of the primary motor cortex improved the functional gait performance and static balance in children with cerebral and was capable of favoring the maintenance of gain one month following the intervention completion.
The study performed by Grecco et al.[16] determined the results of one session of tDCS applied to the primary motor cortex reflected positive changes in gait velocity and static balance in children with cerebral palsy, only one session of tDCS was capable to cause significant increase in step length and gait velocity, after tDCS stimulation compared to the control group (tDCS sham), although these results did not maintain for more than twenty minutes after the stimulation was complete. This finding suggested that as the motor cortex was only stimulated for twenty minutes during rest and there was a momentary increase in cortex activation, which exerted an influence on motor control and gait.
The results of this study agreed with Kashi et al.[17] who found that the application of anodal tDCS stimulation for 15 minutes of prefrontal or primary motor cortex before walking on a moving platform either active or sham tDCS stimulation, improved the postural control and gait velocity in comparison to the placebo group. These results showed that anodal tDCS is capable to cause changes in the excitability of motor cortex, thereby favoring motor control and lower limb movements potentiating locomotor control with a consequent improvement in gait.
Another important finding of this study was that tDCS reflected a long-lasting effect while using tDCS evoked a change in the dysfunctional excitability pattern so that physical therapy can model the cortex activity functional pattern by the activation of specific neural networks and favoring neuroplasticity as reported by Mendonça[11].
Ries et al.[18] agreed with the present study as they concluded that tDCS induces long-lasting changes in the excitability of cortex in both animals and humans. Stimulation is used to modulate the activity of cortex by opening a pathway to prolong, increase, and enhance the functional gains achieved by physical therapy. The combination of physical therapy gait training and tDCS of the primary motor cortex can potentiate the effects on gait and functional performance. The hypothesis is that tDCS leads to maintaining the results after the gait training protocol interruption by making a long-lasting change in the excitability of cortex, thus facilitating the learning process [17].
Grecco et al.[19] reported the benefits of gait training on a treadmill, as they demonstrated positive effects of treadmill training after treatment and during follow up. The benefits included an improvement in functional performance, suggesting that the motor effects can lead to greater independence in children with CP.
Mattern-Baxter [20] reported that treadmill training has led to better results due to the fact that this form of exercise is specific for the different phases of gait and offers controlled, constant velocity, thereby allowing rhythmic strides, believing that this modality of gait training can be added to the physical therapy repertoire of children with cerebral palsy, while the alternative cognitive therapeutics are unacceptable.
In contrast, a study conducted by Willoughby et al. [21] who compared the results of gait training on the ground with treadmill training of the same frequency and duration suggested that treadmill training was not more effective than training on the ground with regard to improved velocity or endurance during gait.
In agreement to the current study Provost et al. [22] suggested that the intensive program of treadmill training 6 days/week for 2 successive weeks can be an effective intervention for ambulatory CP school-aged children causing detectable changes in a positive direction on both a functional gait measure and an endurance measure with significant improvement in energy expenditure and walking velocity were observed,
In concordance with Smania et al.[23] who concluded that repetitive locomotor gait training with an electromechanical treadmill improved spatiotemporal, kinematic gait, endurance, and gait velocity parameters in ambulatory children with diplegic cerebral palsy.
Conclusion:
Based on the findings of the current study both tDCS& treadmill demonstrated positive improvement in selected spatiotemporal gait parameters, gait velocity, and step length, following the different interventions, however, tDCS group had a significant improvement with better results in spatiotemporal gait parameters in comparison with the treadmill. Moreover, anodal tDC stimulation reflected a long-lasting effect that favored the maintenance of the gains for 10 weeks following the studied intervention with further increase in gait velocity and step length.
References
- El-Refaey BH, Abd-El Maksoud GM, Ali OI. Efficacy of feedback respiratory training on respiratory muscle strength and quality of life in children with spastic cerebral palsy: Randomized controlled trial. Bulletin of Faculty of Physical Therapy. 2017 Jan 1;22(1):46.
- Rose S, Guzzetta A, Pannek K, Boyd R. MRI structural connectivity, disruption of primary sensorimotor pathways, and hand function in cerebral palsy. Brain Connectivity. 2011 Oct 1;1(4):309-16.
- Shin YK, Lee DR, Hwang HJ, You SJ, Im CH. A novel EEG-based brain mapping to determine cortical activation patterns in normal children and children with cerebral palsy during motor imagery tasks. NeuroRehabilitation. 2012 Jan 1;31(4):349-55.
- Pitcher JB, Schneider LA, Burns NR, Drysdale JL, Higgins RD, Ridding MC, Nettelbeck TJ, Haslam RR, Robinson JS. Reduced corticomotor excitability and motor skills development in children born preterm. The journal of physiology. 2012 Nov 15;590(22):5827-4
- De Kegel A, Dhooge I, Peersman W, Rijckaert J, Baetens T, Cambier D, Van Waelvelde H. Construct validity of the assessment of balance in children who are developing typically and in children with hearing impairments. Physical therapy. 2010 Dec 1;90(12):1783-94.
- Grecco LA, Tomita SM, Christovão TC, Pasini H, Sampaio LM, Oliveira CS. Effect of treadmill gait training on static and functional balance in children with cerebral palsy: a randomized controlled trial. Brazilian journal of physical therapy. 2013 Feb;17(1):17-23.
- Mattern-Baxter K, Bellamy S, Mansoor JK. Effects of intensive locomotor treadmill training on young children with cerebral palsy. Pediatric physical therapy. 2009 Dec 1;21(4):308-18.
- Dinomais M, Lignon G, Chinier E, Richard I, TerMinassian A, Tich SN. Effect of observation of simple hand movement on brain activations in patients with unilateral cerebral palsy: an fMRI study. Research in developmental disabilities. 2013 Jun 1;34(6):1928-37.
- Grecco LA, Duarte ND, de Mendonça ME, Pasini H, de Carvalho Lima VL, Franco RC, de Oliveira LV, de Carvalho PD, Corrêa JC, Collange NZ, Sampaio LM. Effect of transcranial direct current stimulation combined with gait and mobility training on functionality in children with cerebral palsy: study protocol for a double-blind randomized controlled clinical trial. BMC pediatrics. 2013 Dec;13(1):168.
- Fregni F, Bossio PS, Brunoni AR. Neuromodulaçãoterapêutica: Princípios e avanços da estimulação cerebral nãoinvasivaemneurologia, reabilitação, psiquiatria e neuropsicologia. São Paulo: Sarvier. 2012.
- Mendonça ME, Fregni F. Neuromodulação com estimulação cerebral nãoinvasiva: aplicação no acidente vascular encefálico, doença de Parkinson e dorcrônica. ASSIS, RD Condutaspráticasemfisioterapianeurológica. 2012:307-39.
- Kaski D, Dominguez RO, Allum JH, Bronstein AM. Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: an exploratory study. Neurorehabilitation and neural repair. 2013 Nov;27(9):864-71.
- Adeyemo BO, Simis M, Macea D, Fregni F. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Frontiers in Psychiatry. 2012 Nov 12;3:88.
- Mattern-Baxter K. Effects of partial body weight supported treadmill training on children with cerebral palsy. Pediatricphysical therapy. 2009 Apr 1;21(1):12-22.
- Duarte ND, Grecco LA, Galli M, Fregni F, Oliveira CS. Effect of transcranial direct-current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double-blind randomized controlled trial. PloS one. 2014 Aug 29;9(8):e105777.
- Grecco LA, Duarte NA, Zanon N, Galli M, Fregni F, Oliveira CS. Effect of a single session of transcranial direct-current stimulation on balance and spatiotemporal gait variables in children with cerebral palsy: a randomized sham-controlled study. Brazilian journal of physical therapy. 2014 Oct;18(5):419-27.
- Kaski D, Quadir S, Patel M, Yousif N, Bronstein AM. Enhanced locomotor adaptation aftereffect in the “broken escalator” phenomenon using anodal tDCS. Journal of neurophysiology. 2012 Feb 8;107(9):2493-505.
- Ries L G K, MichaelsenSoares P S A, Monteiro V C and Allegretti K M G: Adaptação cultural e análise da confiabilidade da versãobrasileira da Escala de EquilíbrioPediátrica (EEP). Rev Bras Fisioter. 2012; 16: 205–215.
- Grecco LA, Zanon N, Sampaio LM, Oliveira CS. A comparison of treadmill training and overground walking in ambulant children with cerebral palsy: randomized controlled clinical trial. Clinical rehabilitation. 2013 Aug;27(8):686-96.
- Mattern-Baxter K. Locomotor treadmill training for children with cerebral palsy: Orthopaedic Nursing. 2010; 29(3):169–173.
- Willoughby KL, Dodd KJ, Shields N, Foley S. Efficacy of partial body weight–supported treadmill training compared with overground walking practice for children with cerebral palsy: a randomized controlled trial. Archives of physical medicine and rehabilitation. 2010 Mar 1;91(3):333-9.
- Provost B, Dieruf K, Burtner PA, Phillips JP, Bernitsky-Beddingfield A, Sullivan KJ, Bowen CA, Toser L. Endurance and gait in children with cerebral palsy after intensive body weight-supported treadmill training. Pediatric Physical Therapy. 2007 Apr 1;19(1):2-10.
- Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, Werner C, Bisoffi G, Geroin C, Munari D. Improved gait after repetitive locomotor training in children with cerebral palsy. American journal of physical medicine &rehabilitation. 2011 Feb 1;90(2):137-49.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
