Antimicrobial susceptibility pattern isolated from different clinical samples in Baghdad hospitals
Waleed K. Abdulsahib1*, Osama Q. Fadhil2, Sattar J. Abood1
1 Pharmacology Department, College of Pharmacy, Al Farahidi University, Baghdad, Iraq, 2 Department of Pharmacy, Al Safwa University College, Karbala, Iraq.
Correspondence: Waleed K. Abdulsahib, Pharmacology Department, College of Pharmacy, Al Farahidi University, Baghdad, Iraq. Email: waleedkalel22 @ yahoo.com.
|
ABSTRACT Background: The development of bacteria resistant to the antimicrobial (AM) in hospitals and other health care settings is the main concern of public health. Great AM consumption chiefly in hospitals frequently defined as the most vital factor leading to AM resistance. This study aimed to know the most common bacteria found in our hospitals, most susceptible and resistant AM agents. Patient and Method: This study was done in 10 hospitals of the western half of Baghdad (Al-Karkh side) and continues for two months. In this study, 3055 samples were collected (1718 males & 1337 females) and examined for detection of AM resistance by cultural methods. Culture samples testing directly using Vitek 2 that give dependable proof of identity and susceptibility outcomes after 18–24 h for strains. Results: Study revealed about 38% of samples were taken from blood & about 39% of samples were taken from urine, also about 32% of urine & about 13.7% of blood samples have growth. The main bacteria isolated in urine cultures were 37.5 % Escherichia coli (E. coli), 15.3 %, Klebsiella and Pseudomonas aeruginosa (Ps. aeruginosa) 14.5 %, on the other hand, about 24 % of blood culture isolations were Staphylococcus aureus (S. aureus), 23.5 % Staphylococcus Albus (S. Albus), 14.5% Ps. aeruginosa and 12 % Escherichia coli (E. coli). The main sensitive AM in urine samples are meropenem (91%), on the other hand, the main resistant AM is cefepime (85 %), in blood culture the main important sensitive AM is meropenem (84%), on the other hand, 79.5% of cefotaxime was resistance. Conclusion: The main bacteria isolated in a urine culture is E. coli, while S. aureus is the main bacteria isolated in blood culture. Also, the present study revealed the more effective antimicrobial for urine bacteria are: meropenem, amikacin, and nitrofurantoin and the more efficient AM for blood bacteria are meropenem, amikacin, and ciprofloxacin. Keywords: culture sensitivity test, bacterial resistance and antimicrobial |
Introduction
Multifarious interactions concerning several biological, sociological, and psychological factors trigger AM drugs use. Careful consideration in hospitals and the community must be done due to a serious worldwide problem which is AM resistance that increasing significantly and has consequences for morbidity, mortality, and health care [1]. AM abuse is the main cause of the emergence of resistance to AM agents [2]. In the community, there is a great indication of substandard use of AM. This comprises authorize the use for incorrect conditions and the use of insufficient treatment periods and sub-curative doses. All of these are probable to give reason to the development and broaden AM resistance [3]. Bacterial infections that had been willingly cured by AM are lasting longer due to resistance emergence and this will lead to intense morbidity and mortality [4]. Furthermore, the extra cost of healthcare and the basis of resistant bacteria and/or resistance-encoding genes for patients admitted to the hospitals for curing of infections caused by resistant pathogens [5]. Reduction of AM resistant bacteria in hospitals can be done by Lessen drugs rate use for which there is a resistance, lessen transmission between patients and from hospital staff to patients by successful infection control, rising patients turnover rate, lessen patients entrance have resistant bacteria into hospitals and adding AM for which there is no resistance [6]. Frequency reduction of resistant bacteria in hospitals and total reduction of these infections rate if the above measures made correctly. Furthermore, and most essentially, the effects of their execution should be apparent over a comparatively short time. [7] Culture and sensitivity test (CST): is the assembly of urine, blood or other body fluid samples cultured in a suitable medium and investigated for sensitivity. CST is an analytical lab technique used to recognize the bacteria type and to conclude which AM can efficaciously combat an infection [8]. The nonexistence of bacteria does not denote there is no infection, as it could be a virus that will not propagate in a definite culture medium [9]. The culture of blood samples is done with straightforward blood draw made after the skin is applied with an alcohol pad, then mark messily with a suitable antibacterial solution [10]. This attentive skin cleansing is essential because it stops blood contamination. However, false-positive results of blood are contaminated. [11] A culture of the urine is a way to cultivate and detect bacteria that may be in your urine. The sensitivity test aids medical staff choice the desirable medicine to treat your infection. The urine sample must be collected freshly. [12] In current laboratories, a description of the genome usually used for bacteria identifying. [4]
Aim of the Study:
To know the most common bacteria found in our hospitals and the resistance of each bacteria; to know the percentage of the sensitivity of AMs and the percentage of resistance; to draw a plan for AM selections and to establish the basement for AM requirement in our hospitals.
Materials and Methods:
Study Population
This study was done in 10 hospitals of Al-Karkh medical office (Al Kadhimiya teaching hospital, Al- Kadhimiya pediatric hospital, Central teaching hospital of pediatric, Al-Mahmoudia hospital, Al-Karkh general hospital, Al Karama teaching hospital, Ibn Albitar specialized center for cardiac surgery, Al-Yarmouk Teaching hospital, Abi-Gareeb general hospital, and Al-Karkh obstetric hospital. The data was collected with the assistance of clinical pharmacists and laboratory staff of our hospitals, and the samples were taken from blood, urine, sputum, ear, cerebrospinal fluid (CSF) and others. The study continues for two months from 1/7/2011 to 1/9/2011, and the total numbers of samples were 3055 (1718 males and 1337 females), and (54% child and 46% adults). About (668) isolation of bacteria was found, (4276) AM disks were used and the growth or no growth results were established. Approval was obtained from the institutional ethical committee prior study of 3305 patients admitted to the hospital wards during the work period.
Result
This study was done in ten hospitals of Baghdad Al-Karkh, 3055 samples were collected divided into 1337 female &1718 male samples as shown in figure (1). About 46% of samples are taken from an adult while 54% are child samples as shown in figure (2). Samples were taken from urine, stool, nasal, wound, ear, sputum, (CSF), peritoneal fluid, blood &others. This study revealed 38% were blood samples & about 39% were urine samples as shown in figure (3). This study revealed there is about 32% of urine culture samples present with growth & about 13.7% of blood culture samples present with growth as illustrated in figure (4). The main bacteria isolated in cultures were nine types: about 38% were E. coli, 15.3 % Klebsiella and 14.5 % Pseudomonas aeruginosa (Ps. aeruginosa) in urine of cultures, on the other hand, about 24 % of blood culture isolations was Staphylococcus aureus(S .aureus), 23.5 % Staphylococcus Albus (S. Albus), 14.5 Ps. aeruginosa and 12 % E. coli as demonstrated in figure (5). Thirty-five AM disc types of urine culture were used in this study, in which the main important AMs are shown in figure (6 & 7) below. This study show 91% of meropenem, 85% of amikacin, 78% of nitrofurantoin, 66% of ciprofloxacin, 62% of vancomycin, 58% of gentamicin are sensitive, on the other hand, 85 % of Cefepime, 70% of ampicillin, 68 % of co-trimoxazole & piperacillin, 65% of cephalothin, 57% of ceftriaxone & 53% of cefotaxime are resistance. Thirty-five AM disc types of used for studying blood culture, in which main important AM are 15 as shown in figure (8& 9) below: 84% of meropenem, 82% of amikacin, 74% of ciprofloxacin,71.6% of vancomycin, 64% of clindamycin, 63% gentamicin & ceftazidime were sensitive, on the other hand, 79.5% of cefotaxime, 74.5% of ampicillin, 71.6% of azithromycin, 70% of co-trimoxazole, 63% of piperacillin & 61.4% of cephalothin were resistance.
|
Table 1: Illustrate the main bacteria strains and main AM (sensitive &resistant) in both urine &blood culture, n= 1395. |
|
|||||||
|
|
percent |
Resistant AB |
percent |
Sensitive AB |
percent |
Bacteria Strain |
Sample Type |
|
|
|
85% |
Cefepime |
91% |
Meropenem |
37% |
E. coli |
Urine
|
|
|
|
70% |
Ampicillin |
85% |
Amikacin |
15% |
Klebsiella |
|
|
|
|
68% |
Co- trimoxazole |
78% |
Nitrofurantoin |
14.5% |
Ps. aeruginosa |
|
|
|
|
79.5% |
Cefotaxime |
84% |
Meropenem |
24% |
St. aureus |
Blood
|
|
|
|
74.5% |
Ampicillin |
82% |
Amikacin |
23.5% |
St. albus |
|
|
|
|
70% |
Azithromycin |
74% |
Ciprofloxacin |
14.5% |
Ps. aeruginosa |
|
|
The main AM dispensed in our hospitals during the study period (i.e. 1/7/2011-1/9/2011) were 21% ceftriaxone, 25.5% cefotaxime, 38% ampicillin plus cloxacillin, 23% ciprofloxacin, 4% meropenem& vancomycin, 2% amikacin &1% nitrofurantoin as shown in figure (10).
Discussion
A CST is an indicative laboratory technique used to detect the bacteria kind and to conclude which AM can effectively combat an infection [8] (Shapiro et al; 2006). Urine culture consists of 39% of this study, while blood culture consists of 38%, on the other hand, there is a low percentage of other cultures types like CSF, wound& nasal swabs in comparison with urine & blood cultures of this study. This means only a simple sampling of cultures is done in our laboratories while the difficult procedure was ignored. After the sample collection, the percent of growth were low in blood &urine culture samples (32% growth in urine, 13.7% growth in blood cultures) while in the other counters the percent is either lower as in a Nigerian Tertiary Hospital (18.2%) [13] or higher as in south part of India (47.5%) [14]. This means the procedure in our laboratories needs more precise techniques or the samples drown when the patient already on the AM regimen. Urine culture results shown E. coli is the main strain were found (37.5%), Klebsiella was the second strain isolated (15.3%), and Ps. aeruginosa was the third strain obtained in this study, these results were nearly similar to the result obtained by Al-Marzoqi [15]. Blood culture result shown S. aureus is the main strain were found (24%), S. Albus is the second strain (23.5%), and Ps. aeruginosa (14.5%) is the third strain in this study, these result which is similar to the study done by Al-Taie coworkers in Baghdad Hospitals [16]. AM discs used in our laboratories are in huge quantity, we notice about 35 types of AM discs, while we need only about 15 discs for preparing culture and sensitivity tests, and this requirement mentioned in the FDA chart [15]. The suggested groupings of AM agents with FDA clinical indications not followed by our technician, this means wasting in money, time and effort, example on this FDA chart suggested grouping: using ceftazidime, gentamicin, tobramycin and piperacillin for Ps. aeruginosa while in our laboratories use amoxicillin plus clavulanic acid, trimethoprim, oxacillin, and ampicillin. In urine culture samples the more efficient AM were meropenem (91%), amikacin (85%) [15], nitrofurantoin (78%), ciprofloxacin (66 %), vancomycin (62 %), gentamicin (58 %)(nearly parallel to the result obtained by [17] and [18] , on other hand the ineffective AM (resistant) were Cefepime (85%) [19], ampicillin (75%)[20] , co-trimoxazole(68%) [21]. The above result show meropenem is more sensitive in comparison with other AM due to high cost and limited use of meropenem, on the other hand, amikacin and nitrofurantoin have a good susceptibility due to their limited use in our hospitals [22]. Also, the existing urine samples show there is increasing in the resistance of the 3ed generation cephalosporin's (especially cefotaxime and ceftriaxone) [23], ampicillin, piperacillin and co- ampicillin, piperacillin, and Co-trimoxazole due to unreasonable use of these AM [24]. Amikacin besides nitrofurantoin are relatively cheap, easily available drugs and highly efficient when compared with other AM that have low efficacy and higher cost and less available in our hospitals. In blood culture samples the more efficient AM were meropenem (84 %), amikacin (82 %), ciprofloxacin (74 %), vancomycin (71.6 %), clindamycin (64 %) gentamicin (63%), ceftazidime (63 %) (these result concomitant with Bell and co-workers results (2005), on the other hand, the main resistant AM are cefotaxime (79.5%), ampicillin (74.5%), azithromycin (71%) and co-trimoxazole (70%), these results revealed the good efficacy of meropenem, amikacin, and ciprofloxacin due to the high cost of meropenem and decreasing in use of amikacin and ciprofloxacin in our hospitals, these result similar to the neighboring countries like Iran [19]. Also, the current study demonstrated the ineffectiveness of the 3ed generation cephalosporins (especially cefotaxime and ceftriaxone)[23], azithromycin[25], co-trimoxazole[24] and ampicillin [26] for treating the blood bacteria, the results explained by the blind use of AM in the treatment of infection [27]. The main AM dispensed in 10 hospitals during the period of study not concomitant with the strength of bacterial strain isolations, an examples ceftriaxone found (57 %) resistant in urine samples, (52 %) in blood samples while the dispensed was (21 %) of total AM during our study. Another example cefotaxime found (80 %) resistant in blood, (53 %) in urine, while the dispensed was (25 %) of the total AM. Another example amikacin found (82 %) sensitive in blood, (85 %) sensitive in urine while the dispensed (2 %) of the total of AM .The last example is nitrofurantoin, we found (78 %) sensitive in urine while the dispensed was (1%).
Conclusion
Old AM like nitrofurantoin and amikacin are worthy choice due to high sensitivity of most strains of bacteria (especially Klebsiella and St. albus), low cost and availability, in addition to that, good effectiveness against bacteria (especially Ps. aeruginosa and St. aureus) of meropenem and ciprofloxacin should be saved for high resistant bacteria to decrease the emergence of resistance against these new AM.
Acknowledgments
Not applicable
Conflicts of interest
The authors declare that there is no conflict of interest.
Authors' Contributions
All authors designed the experiments. Waleed K. Abdulsahib, Osama Q. Fadhil and Sattar J. Abood performed the experiments. Waleed K. Abdulsahib, Osama Q. Fadhil and Sattar J. analyzed the data. Waleed K. Abdulsahib wrote the manuscript. All authors read and approved the manuscript.
Funding
None.
Data availability
All datasets generated or analyzed during this study are included in the manuscript and/or the Supplementary Files
Ethics Statement
Not applicable.
References
- Franco BE, Martínez MA, Rodríguez MAS, Wertheimer AIJI, resistance d. The determinants of the antibiotic resistance process. 2009;2:
- Organization WH. WHO global strategy for containment of antimicrobial resistance. Geneva: World Health Organization; 2001.
- Watson RL, Dowell SF, Jayaraman M, Keyserling H, Kolczak M, Schwartz B. Antimicrobial use for pediatric upper respiratory infections: reported practice, actual practice, and parent beliefs. Pediatrics. 1999 Dec 1;104(6):1251-7.
- Dean, A., Lee, D. Bedside laboratory and microbiologic procedures, 201
- Gonzales, R., Steiner, J. F., Sande, M. A. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. Jama, 1997; 278(11), 901-904.
- Smith, R., Coast, J. The true cost of antimicrobial resistance. BMJ, 2013; 346, f1493.
- Haber, M., Levin, B. R., Kramarz, P. Antibiotic control of antibiotic resistance in hospitals: a simulation study. BMC infectious diseases, 2010; 10(1), 254.
- Hockberger, R. S., Marx, J. A., & Walls, R. M. (Eds.). Emergency Medicine: Concepts and Clinical Practice. Mosby, 2006.
- Jung CL, Lee M, Chung WSJKJoCM. Clinical evaluation of the multiplex PCR assay for the detection of bacterial pathogens in respiratory specimens from patients with pneumonia. 2010; 13(1):40-6.
- Schwalbe, R., Steele-Moore, L., Goodwin, A. C. Antimicrobial susceptibility testing protocols. CRC Press, 2007.
- Bisno, A. L., Gerber, M. A., Gwaltney Jr, J. M., Kaplan, E. L., Schwartz, R. H. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clinical Infectious Diseases, 2002; 113-125.
- Hooton, T. M., Bradley, S. F., Cardenas, D. D., Colgan, R., Geerlings, S. E., Rice, J. C., ... Nicolle, L. E. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical infectious diseases, 2010; 50(5), 625-663.
- Tamuno, I., Fadare, J. O. Drug prescription pattern in a Nigerian tertiary hospital. Tropical Journal of Pharmaceutical Research, 2012; 11(1), 146-152.
- Bhargavi, P. S., Rao, G. T., Mukkanti, K., Kumar, D. B., Krishna, T. P. The increasing emergence of antibacterial resistance mainly in uropathogens: southeast part of India. International journal of microbiology research, 2010; 2(1), 1.
- Al-Marzoqi, A. H. Etiology and antimicrobial sensitivity of common uropathogens in Hilla infants. Medical Journal of Babylon, 2008; 5(3-4), 579-586.
- Al-Taie, L. H. Isolation and identification of bacterial burn wound infection and their sensitivity to antibiotics. Al-Mustansiriyah Journal of Science, 2014; 25(2), 17-24.
- Shaikh, S., Fatima, J., Shakil, S., Rizvi, S. M. D., Kamal, M. A. Antibiotic resistance and extended-spectrum beta-lactamases: Types, epidemiology, and treatment. Saudi journal of biological sciences, 2015; 22(1), 90-101.
- Góngora Rubio F, Oliveira VDC, Rangel RMC, Nogueira MCL, Almeida MTGJBJoID. Trends in bacterial resistance in a tertiary university hospital over one decade. 2013;17(4):480-2.
- Bechashk, S. M., Moradi, G., Mohsenpour, B., Ramazanzadeh, R. Prevalence of Cefepime-Resistant Escherichia coli in Iran: A Meta-Analysis (2007–2016). Iranian journal of public health, 2019; 48(4), 603.
- Kim, D., Lee, H., Yoon, E. J., Hong, J. S., Shin, J. H., Uh, Y., ... Jeong, S. H. Prospective Observational Study of the Clinical Prognoses of Patients with Bloodstream Infections Caused by Ampicillin-Susceptible but Penicillin-Resistant Enterococcus faecalis. Antimicrobial Agents and Chemotherapy, 2019; 63(7), e00291-19.
- Khalil, M. A., Moawad, S. S., Hefzy, E. M. In vivo activity of co-trimoxazole combined with colistin against Acinetobacter baumannii producing OXA-23 in a Galleria mellonella model. Journal of medical microbiology, 2018; 68(1), 52-59.
- Pitout, J. D., Laupland, K. B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. The Lancet infectious diseases, 2008; 8(3), 159-166.
- Meyer, E., Schwab, F., Schroeren-Boersch, B., Gastmeier, P. The dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Critical care, 2010; 14(3), R113.
- Straus, W. L., Qazi, S. A., Kundi, Z., Nomani, N. K., Schwartz, B., Pakistan Co-trimoxazole Study Group. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus Amoxycillin for pneumonia among children in Pakistan: randomized controlled trial. The Lancet, 1998; 352(9124), 270-274.
- Chetchotisakd, P., Chaowagul, W., Mootsikapun, P., Budhsarawong, D., Thinkamrop, B. Maintenance therapy of melioidosis with ciprofloxacin plus azithromycin compared with cotrimoxazole plus doxycycline. The American journal of tropical medicine and hygiene, 2001; 64(1), 24-27.
- Landman, D. A. V. I. D., Mobarakai, N. K., Quale, J. M. Novel antibiotic regimens against Enterococcus faecium resistant to ampicillin, vancomycin, and gentamicin. Antimicrobial agents and chemotherapy, 1993; 37(9), 1904-1908.
- Gonzales, R., Bartlett, J. G., Besser, R. E., Cooper, R. J., Hickner, J. M., Hoffman, J. R., Sande, M. A. Principles of appropriate antibiotic use for the treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Annals of emergency medicine, 2001; 37(6), 690-697.
Figure 1: The number of male and female specimens in 10 hospitals, n= 3055.
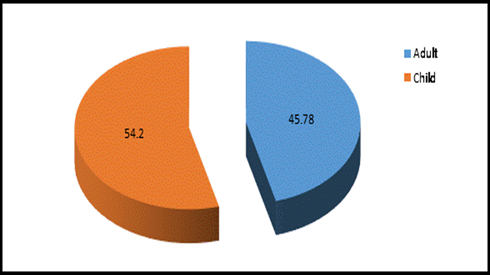
Figure 2: Adult & Child specimens percentage in 10 hospitals, n =3055.
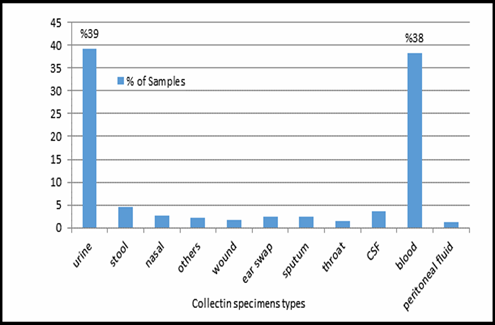
Figure 3: Collecting specimens types in 10 hospitals, n=3055.
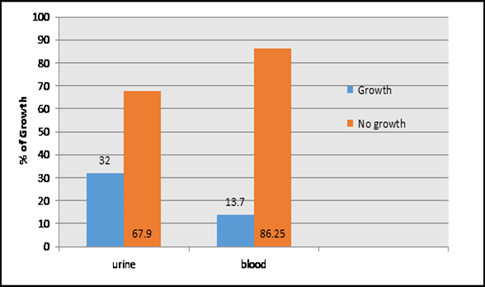
Figure 4: Percent of growth and no growth in urine and blood collecting specimens, n=3055.
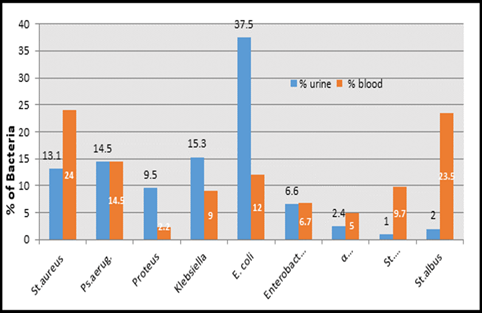
Figure 5: Percent of bacteria species in blood and urine Cultures, n=3055.
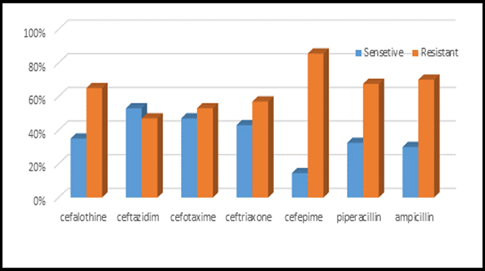
Figure 6: Antibiotics sensitivity & resistance in urine culture samples part A.
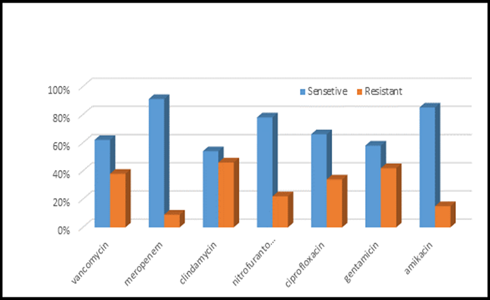
Figure 7: Antibiotics sensitivity & resistance in urine culture samples, part B, n in both part A& B= 977
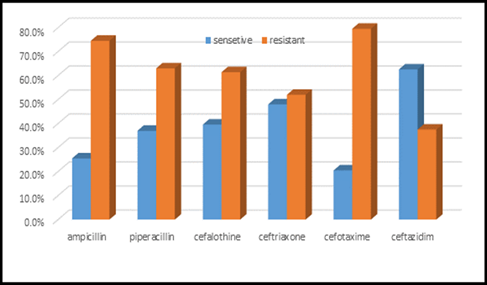
Figure 8: Antibiotic sensitivity & resistance in blood culture samples, part A.
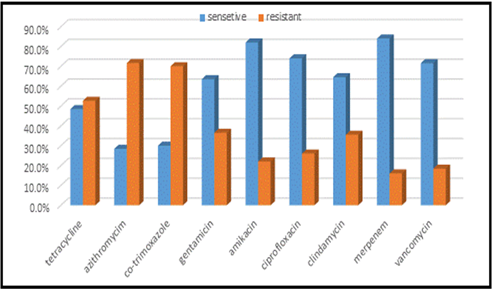
Figure 9: Antibiotics sensitivity & resistance in blood culture samples, part B. n in both A &B= =418.
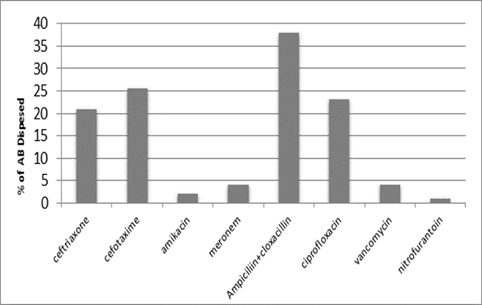
Figure 10: Antibiotics dispensed in 10 hospitals in Baghdad health directorate-Al-Karkh during the period study, n=182530.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
