The effect of some antibiotics on pathogenic characteristics of some bacteria
Ashwaq Hazem Najem, Iman Mahmood Khudhur, D. Adeeba Younis Shareef *
Biology Department, Mosul College of Science, University of Mosul, Iraq
ABSTRACT
Foley catheters are most widely employed in treating urinary tract infection (UTI) during and post-surgical operations. Different isolates of gram-negative bacteria causing UTI were identified and their susceptibility to several antibiotics of different groups was studied. The ability to adhere to catheter surface/biofilm formation was investigated. The effect of the antibiotics as biofilm inhibitors were studied at their sub minimal, minimum, and double the minimum inhibitory concentration (MIC). The results showed variable resistance patterns; the highest resistance was shown by amoxicillin (70%) and the most effective ones were meropenem (MEM; 90%) and ciprofloxacin (CIP; 80%). Enterococcus faecalis was sensitive to the high concentration of MEM with MIC of 278 µg/ml, Pseudomonas aeruginosa was sensitive at 556 µg/ml of MEM, while Escherichia coli and Salmonella typhi were sensitive to CIP at MIC of 2.171 µg/ml. The study of the ability of the bacteria to adhere and biofilm formation on catheter showed different abilities, Morganella morganii and Pseudomonas aeruginosa showing the highest activity. The use of the antibiotics MEM and CIP inhibited the biofilm growth in all bacteria at MIC and sub MIC for CIP except for Aeromonas hydrophila, and MIC and sub MIC for MEM on Pseudomonas aeruginosa. Mixing the MIC concentrations of both MEM and CIP showed a strong effect on the eradication of biofilm in all the bacteria.
Keywords: the effect of some antibiotics, eradication of biofilm, catheters
Introduction
Foley catheters are commonly employed in indwelling urinary catheters and have an essential role in the relief of urinary retention and alleviation of urinary incontinence in the post-surgical procedures.[1] Unfortunately, polymeric medicals are susceptible to be colonized by microorganisms causing infections and hurting patients’ health. [2]
The microorganisms implicated in catheter association urinary tract infections (CAUTIs) are found on catheters predominantly in the form of biofilm, a structure providing them with a protective environment and making them more virulent and less sensitive to antimicrobial therapy.[3] Most antibiotics have low efficacy against biofilm and have adverse effects.[4] The close attention paid to biofilms is due to the prevalence of this form of microbial symbiosis both in the natural environment and in industrial systems.[5] Biofilms are one of the most common forms of bacteria in most natural conditions.[6-8] Biofilms are communities of microorganisms, which are embedded in self-produced extracellular polymeric substances.[9] The prominence of biofilms for human health has stimulated the efforts to recognize the mechanism of biofilm formation to open a new axis of customizing measures highly effective against bacteria and their biofilm forms.[10] Biofilms protect bacteria from traditional antibiotics by representing a mechanical barrier and also reducing bacterial metabolism within the biofilm.[11] Biofilm-related infections are challenging to treat.[12] Biofilms resist many biocides. Thus, biofilm formation inhibiting and decreasing microbial binding is a promising antimicrobial trend.[13] Contamination is a common reason for indwelling medical failure.[14] The microbial colonization in the development of biofilms related to its recalcitrant nature could lead to infection.[15]
The impregnation, combination, or coating of several antibiotics examined and various factors could affect their efficacy. For example, the formulation and physicochemical affinity between the antibiotics and the polymers has been shown to drastically modify the length and rate of antibiotic release [16]. Antimicrobial straitening causes reducing biomaterial infections and considerations [17].
Considering the feasibility of materials selection or modification on controlling adhesion and biofilm formation, the potential antibiotic inhibition of bacterial adhesion and biofilm formation and eradication at MIC concentration and the synergistic effect between them was evaluated as the aim of this research.
Materials and Methods
- Selection of the Isolates
Morganella morganii, Pseudomonas aeruginosa, Escherichia coli 1, Escherichia coli 2, Klebsiella pneumoniae, Aeromonas hydrophila, Salmonella typhi, Enterobacter clocae, Enterococcus faecalis, Proteus mirabilis were the isolated bacteria from urinary tract infection. These bacterial species were identified using standard microbiological procedures including Gram staining, colonial morphology, catalase test, cytochrome oxidase reaction, motility, and other biochemical tests, which were carried out based on Bergey's manual.[18]
- Determination of antibiotic susceptibility:
The evaluation of susceptibility of bacteria to antimicrobial agents was performed according to CLSI, and the standard Kirby-Bauer disc diffusion method was used for the antibiotics listed in Table 1. The antimicrobial resistance of the isolate was investigated. In this method, antibiotic discs were used. The discs were placed on a Mueller-Hinton agar plate (Oxoid) inoculated (0.5) McFarland standard inoculated with 1.5×108 CFU/mL of test microorganism. The zones of inhibition were determined according to Clinical and Laboratory Standards Institute guidelines.[19].
|
Table 1. Antibiotics that are used for the sensitivity test. |
|||
|
Antibiotics |
Conc. Of disc |
Abbreviation |
Producing company |
|
Meropenem |
10 µg |
MEM |
Mast diagnostics (UK) |
|
Gentamicin |
10 mcg |
CN |
Bioanalyse(turkey) |
|
Amoxicillin |
25 mcg |
AX |
bioanalyse(turkey) |
|
Trimethoprime |
10 mcg |
TMP |
bioanalyse(turkey) |
|
Ciprofloxacin |
10 mcg |
CIP |
bioanalyse(turkey) |
|
Cefotaxime |
30 mcg |
CTX |
bioanalyse(turkey) |
|
Nitrofurantoin |
100 mcg |
F |
bioanalyse(turkey) |
- In vitro adhesion of bacteria to Nelaton catheter:
The catheter was cut to ca. 0.5 cm2 fragments in Petri dishes. Adhesion to catheter was investigated in vitro by TTC (2,3,5-triphenyltetrazolium chloride).[20] The bacterial suspensions were standardized to 0.5McFarland standard in sterile PBS (phosphate-buffered saline)[19, 21] and incubated with catheter for 2h (35-37 ºC).
- In vitro determination of biofilm formation by bacteria on Nelaton catheter:
The capacity of the formation of biofilm by isolates on the catheter was studied in vitro by TTC. Standardized bacterial suspensions (0.5 McFarland standard) in MHB were incubated with 0.5 cm2 catheter for 24 h at 35-37ºC. Non-adherent cells were eliminated by careful rinsing of catheter fragment with sterile PBS and then re-suspended in fresh MHB. One drop of 1% TTC was added and incubated overnight. Biofilm formation was determined by red formazan precipitates on the catheter surface and in the medium. The minimal antibiotic concentration that eradicated the mature biofilm was determined visually by the lack of red formazan precipitates.[22]
- In vitro effect of antibiotics on adhesion and biofilm formation by bacteria on Nelaton catheter:
This assay was based on the TTC method described above. In each experiment, several concentrations of antibiotics were used (0.5MIC, MIC, 2MIC). (I) Standardized bacterial suspensions in sterile PBS containing relevant antibiotics were incubated with the catheter at 35-37ºC. A drop of 1% TTC was added and incubated overnight at 35-37ºC. (II) Bacterial suspensions in MHB containing antibiotics were incubated with the appropriate piece of the catheter for 72h at 35-37ºC. Then, the medium was changed and the catheter was washed. A drop of 1% TTC was added and incubated overnight at 35-37ºC. (III) Mature 72-h biofilms were incubated in the presence of antibiotics for 24h at 35-37ºC. The minimal concentration of antibiotic that eradicated mature biofilm was determined by inhibition of red formazan precipitation, both on catheter surface and in the medium.
Impregnation:
Briefly, catheter segments (0.5 cm in length) were impregnated with a mixture of ciprofloxacin (0.5MIC, MIC, 2MIC) and Meropenem (0.5MIC, MIC, 2MIC) for an additional 24h. post impregnation, catheters were air-flushed, dehydrated overnight, washed, and dried again. Also, controls were tested for comparison.[23] All assays were done with three replicates.
Results and Discussion
- Antibiotic susceptibility:
Table 2 shows the antibiotic susceptibility and Table 3 shows susceptibility patterns for bacterial species used in this study. High resistance patterns were observed against the used antibiotics except for meropenem with amoxicillin (70%) followed by cefotaxime, gentamicin, and trimethoprim (50%). The lowest resistance was against meropenem with 90% sensitivity followed by ciprofloxacin (80% sensitivity). Results revealed that infections are difficult to treat and require complex multi-drug treatment strategies, particularly when biofilms are poly microbial.[24]
|
Table 2: Inhibition zones of antibiotic sensitivity test (mm). |
|||||||
|
Bacterial species |
MEM |
CTX |
AX |
TMP |
F |
CIP |
CN |
|
E. coli 1 |
50 |
0 |
0 |
0 |
16 |
25 |
10 |
|
E. coli 2 |
40 |
0 |
0 |
0 |
28 |
18 |
12 |
|
A. hydrophila |
40 |
30 |
0 |
30 |
30 |
30 |
20 |
|
K. pneumoniae |
20 |
11 |
0 |
0 |
20 |
9 |
11 |
|
Ps. aeruginosa |
38 |
9 |
0 |
17 |
0 |
40 |
26 |
|
P. mirabilis |
14 |
19 |
8 |
8 |
16 |
34 |
14 |
|
E. clocoae |
28 |
7 |
0 |
28 |
10 |
36 |
0 |
|
M. morganii |
44 |
24 |
0 |
20 |
16 |
34 |
18 |
|
E. feacalis |
55 |
18 |
38 |
0 |
36 |
30 |
8 |
|
S. typhi |
40 |
33 |
35 |
35 |
30 |
30 |
14 |
|
Table 3: Antibiotic susceptibility patterns. |
|||||||
|
Bacterial species |
MEM |
CTX |
AX |
TMP |
F |
CIP |
CN |
|
E. coli 1 |
S |
R |
R |
R |
I |
S |
R |
|
E. coli 2 |
S |
R |
R |
R |
S |
I |
R |
|
A. hydrophila |
S |
S |
R |
S |
S |
S |
S |
|
K. pneumoniae |
S |
R |
R |
R |
S |
R |
R |
|
Ps. aeruginosa |
S |
R |
R |
S |
R |
S |
S |
|
P. mirabilis |
I |
I |
R |
R |
I |
S |
I |
|
E. clocoae |
S |
R |
R |
S |
R |
S |
R |
|
M. morganii |
S |
S |
R |
S |
I |
S |
S |
|
E. feacalis |
S |
I |
S |
R |
S |
S |
R |
|
S. typhi |
S |
S |
S |
S |
S |
S |
I |
CAUTIs were mainly caused by gram-negative bacteria resistant to common antibiotics. For this reason, research should be done on CAUTIs including effective prevention, and understanding of antimicrobial resistance mechanisms, and the expansion of new antibiotics for patient safety.[25] Therapy combination is suggested as a novel strategy to maximize the antimicrobial efficacy against pathogens and suppress the spread of resistance. [26] Unfortunately, developing new antibiotics faces many challenges. Alternative approaches to control bacterial infections are urgently required. [27]
- Determination of MIC value:
In this study, meropenem and ciprofloxacin were chosen to detect their effects on adhesion and biofilm formation and determination of MIC value (as shown in Tables 4 and 5). For CIP the highest MIC value was for E. coli 1 and Salmonella typhi and for MEM the highest MIC value was for Morganella morganii. The organisms that caused CAUTIs were gram-positive/negative bacteria and yeasts and were capable of adhering to surfaces and forming biofilms. Plank tonic or free-floating biofilm can be found in the urothelium, prostate stones, and implanted foreign bodies. The biofilm protects the bacteria from exposure to innate immune defenses and antibiotic treatments, thus promotes the diffusion of drug resistance markers and other virulence factors.[28]
|
Table 4: MIC values for CIP. |
|||||||||
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
|
Bacterial species |
1512µ/ml |
556 µ/ml |
278 µ/ml |
139 µ/ml |
69.5 µ/ml |
34.75 µ/ml |
17.375 µ/ml |
8.687 µ/ml |
4.343 µ/ml |
|
E. coli 1 |
|
|
|
|
|
|
|
|
+ |
|
A. hydrophila |
|
|
|
|
|
+ |
|
|
|
|
Ps. aeruginosa |
|
|
+ |
|
|
|
|
|
|
|
M. morganii |
|
|
|
|
|
|
+ |
|
|
|
E. faecalis |
|
|
|
+ |
|
|
|
|
|
|
S. typhi |
|
|
|
|
|
|
|
|
+ |
Stock solution: 1gm in 10 ml D.W.
Biofilms require dynamic preventive and control measures. Biofilm research will lead to a better understanding of the disease process and will subsequently lead to the development of new prevention and treatment options. A perfect approach will include a combination of antibiofilm molecules, with an anti-bacterial effect, active at sub-inhibitory concentrations to reduce the risk of developing resistance and with low toxicity for the host cells.[24]
|
Table 5: MIC values for MEM. |
||||||||
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
Bacterial species |
556 µ/ml |
278 µ/ml |
139 µ/ml |
69.5 µ/ml |
34.75 µ/ml |
17.375 µ/ml |
8.687 µ/ml |
4.343 µ/ml |
|
E. coli 1 |
|
|
|
+ |
|
|
|
|
|
A. hydrophila |
|
|
+ |
|
|
|
|
|
|
Ps. aeruginosa |
|
|
|
+ |
|
|
|
|
|
M. morganii |
|
|
|
|
|
|
+ |
|
|
E. faecalis |
|
+ |
|
|
|
|
|
|
|
S. typhi |
|
|
|
+ |
|
|
|
|
Stock solution: 1gm in 10 ml D.W.
Table 6 shows different results about bacterial ability to adhere to catheter segments after 24 hrs. Morganella morganii showed a strong ability to adhere, followed by E. coli 1. Pseudomonas had a moderate ability to adhere. The other bacterial species had a low ability to adhere to catheter segments, except E. coli 2 which showed no ability to adhere. These results were according to the red color of formazan formed after the addition of TTC on catheter surface and medium.[29]
|
Strong adhesion(+++), Moderate(++), Weak adhesion (+) |
|
Table 6: Adhesion results (formation of formazan after 24 hours). |
|
|
Bacterial species |
Adhesion |
|
E. coli 1 |
++ |
|
E. coli 2 |
- |
|
A. hydrophila |
+ |
|
K.pneumoniae |
+ |
|
Ps.aeruginosa |
++ |
|
P. mirabilis |
+ |
|
E. clocoae |
+ |
|
M. morganii |
+++ |
|
E. faecalis |
+ |
|
S. typhi |
+ |
Table 7 shows various results of bacterial ability to form a biofilm. E. coli1, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus clocoae, Morganella morganii, Enterococcus feacalis, and Salmonella typhi had an excellent capacity to form biofilm after three days of incubation with the addition of fresh media every 24 hr, while other bacterial species had moderate ability to form biofilm according to the appearance of red formazan precipitate on catheter segments. Tetrazolium salts have been used previously to study cell growth and biofilm formation in various bacterial models.[30] If bacteria win the competition, the implant surface would eventually become covered by biofilm, and tissue cell functions will be impaired by bacterial toxins.[31]
|
Table 7: Biofilm formation after three days. |
|
|
Bacterial species |
Biofilm formation |
|
E. coli 1 |
+++ |
|
E. coli 2 |
++ |
|
A. hydrophila |
++ |
|
K. pneumoniae |
++ |
|
Ps. aeruginosa |
++++ |
|
P. mirabilis |
+++ |
|
E. clocoae |
+++ |
|
M.morganii |
+++ |
|
E. faecalis |
+++ |
|
S. typhi |
+++ |
Strong biofilm forming(+++), Moderate (++), Weak biofilm forming(+)
We chose six bacterial species from the bacteria under study according to their sensitivity to MEM and CIP. The effect of 0.5MIC, MIC, and 2MIC for both antibiotics was studied on the ability of bacterial adhesion (Tables 8 and 9). The chosen concentrations showed a high ability to inhibit bacterial adhesion on catheters segments (Figures 1). Also, the bacterial species lost their ability to adhere after soaking catheter fragments in the MIC values for both antibiotics together.
Tables 10 and 11 show the effect of 0.5MIC, MIC, and 2MIC for both antibiotics on the eradication of formed biofilm. 2MIC value showed high efficiency to prevent biofilm formation for all studied bacterial species as shown in tables for both antibiotics. The results of MIC values were similar in effect except for A. hydrophila which retained the ability to form biofilm when using CIP and Ps. aeruginosa when using MP. Also, 0.5MIC value for CIP did not affect A. hydrophila's ability to form a biofilm. The same results were observed with 0.5MIC value for the effect of MEM on biofilm formation ability of Ps. aeruginosa and M. morganii.
|
Table 8: Adhesion results for CIP and impregnation. |
|||||||
|
Bacterial species |
2MIC |
MIC |
½ MIC |
CIP MP MIC+ MIC |
|||
|
E. coli 1 |
8.687 µ/ml |
- |
4.343 µ/ml |
- |
2.171 µ/ml |
- |
- |
|
A. hydrophila |
69.5 µ/ml |
- |
34.75 µ/ml |
- |
17.375 µ/ml |
- |
- |
|
Ps. aeruginosa |
278 µ/ml
|
- |
139 µ/ml |
- |
69.5 µ/ml |
- |
- |
|
M. morganii |
34.75 µ/ml |
- |
17.375 µ/ml |
- |
8.687 µ/ml |
- |
- |
|
E. faecalis |
278 µ/ml |
- |
139 µ/ml |
- |
69.5 µ/ml |
- |
- |
|
S. typhi |
8.687 µ/ml |
- |
4.343 µ/ml |
- |
2.171 µ/ml |
- |
- |
(-) No growth and clearance of the medium
|
Table 9: Adhesion results for MEM and impregnation. |
||||||||||||
|
Bacterial species |
2MIC |
MIC |
½ MIC |
CIP MP MIC+ MIC |
|
|||||||
|
E. coli 1 |
139 µ/ml |
- |
69.5 µ/ml |
- |
34.75 µ/ml |
- |
- |
|
||||
|
A. hydrophila |
278 µ/ml |
- |
139 µ/ml |
- |
69.5 µ/ml |
- |
- |
|
||||
|
Ps. aruginosa |
139 µ/ml |
- |
69.5 µ/ml |
- |
34.75 µ/ml |
- |
- |
|
||||
|
M. morganii |
17.375 µ/ml |
- |
8.687 µ/ml |
- |
4.343 µ/ml |
- |
- |
|
||||
|
E. faecalis |
556 µ/ml |
- |
278 µ/ml |
- |
139 µ/ml |
- |
- |
|
||||
|
S. typhi |
139 µ/ml |
- |
69.5 µ/ml |
- |
34.75 µ/ml |
- |
- |
|
||||
(-) No growth and clearance of the medium.
The results revealed that mixing MIC values of both antibiotics and soaking the catheter segments is highly effective in eradication and prevention of biofilm formation after three days of incubation with changing the media and washing catheter segments without the appearance of formazan red color after adding TTC at the end of third-day. The combination of antibiotics with dispersal agents is a promising alternative strategy to treat biofilm-forming infections. Indeed, increasing antibiotic susceptibilities observed with dispersal agents.[32]
Causative pathogens and antibiotic resistance concerning resistance profile among biofilm-producing bacterial strains, Meropenem, and Ciprofloxacin are good therapeutic options. Catheterization under aseptic condition, frequent catheter change, early treatment of urinary infection, and proper patient education on catheter hygiene are some of the methods.[33]
|
Table 10: Biofilm eradication results for CIP MIC and impregnation. |
|||||||||||
|
Bacterial species |
2MIC |
MIC |
½ MIC |
CIP MEM MIC+ MIC |
|||||||
|
E. coli 1 |
8.687 µ/ml |
- |
4.343 µ/ml |
- |
2.171 µ/ml |
- |
- |
|
|||
|
A. hydrophila |
69.5 µ/ml |
- |
34.75 µ/ml |
+ |
17.375 µ/ml |
+ |
- |
|
|||
|
Ps. aeruginosa |
278 µ/ml |
- |
139 µ/ml |
- |
69.5 µ/ml |
- |
- |
|
|||
|
M. morganii |
34.75 µ/ml |
- |
17.375 µ/ml |
- |
8.687 µ/ml |
- |
- |
|
|||
|
E. faecalis |
278 µ/ml |
- |
139 µ/ml |
- |
69.5 µ/ml |
- |
- |
|
|||
|
S. typhi |
8.687 µ/ml |
- |
4.343 µ/ml |
- |
2.171 µ/ml |
- |
- |
|
|||
|
Table 11: Biofilm eradication results for MEM MIC and impregnation. |
|||||||||
|
Bacterial species |
2MIC |
MIC |
½ MIC |
CIP MEM MIC+ MIC |
|
||||
|
E. coli 1 |
139 µ/ml |
- |
69.5 µ/ml |
- |
34.75 |
- |
- |
|
|
|
A. hydrophila |
278 µ/ml |
- |
139 µ/ml |
- |
69.5 µ/ml |
- |
- |
|
|
|
Ps. aeruginosa |
139 µ/ml |
- |
69.5 µ/ml |
+ |
34.75 µ/ml |
+ |
- |
|
|
|
M. morganii |
17.375 µ/ml |
- |
8.687 µ/ml |
- |
4.343 µ/ml |
+ |
- |
|
|
|
E. faecalis |
556 µ/ml |
- |
278 µ/ml |
- |
139 µ/ml |
- |
- |
|
|
|
S. typhi |
139 µ/ml |
- |
69.5 µ/ml |
- |
34.75 µ/ml |
- |
- |
|
|
(-) No growth and clearance of the medium.
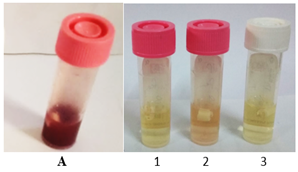
Figures 1: Biofilm eradication result of E. faecalis. A: The control tube (The catheter and bacteria forming biofilm with the appearance of red color due to formazan forming after addition of TTC); 1: Tube containing 556µ/ml meropenem; 2: Tube containing 278µ/ml meropenem; 3: Tube containing 139µ/ml meropenem.
Conclusions
The results of the study showed variable resistance patterns to the selective antibiotic and the most effective ones were meropenem (MEM; 90%) and ciprofloxacin (CIP; 80%). Morganella morganii and Pseudomonas aeruginosa showed the highest activity to adhere and biofilm formation on catheter among the studied bacterial species which had different abilities in mixing the MIC concentrations of both antibiotics. MEM and CIP revealed a strong effect on the eradication of biofilm in all the bacteria.
References
- Colletta, A., Wu, J., Wo, Y., Kappler, M., Chen, H., Xi, C., Meyerhoff, M.S Nitroso N acetylpenicillamine (SNAP) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device To Prevent Catheter-Associated Urinary Tract Infections. (ACS Biomater. Sci 2015; (1). 416−424.
- Haraga, I., Abe, S., Jimi, S., Kiyomi, F., Yamaura, K. Increased biofilm formation ability and accelerated transport of Staphylococcus aureus along a catheter during reciprocal movements. J. of Microbiol. Methods. 2016; 132:63-68.
- Trautner, W, Darouiche, O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control. 2004; 32(3):177-8
- Shenderovich, G., Zaksb, B., Kirmayera, D., Lavyc, E., Steinbergb, D. Michael Friedmana. Chlorhexidine sustained-release varnishes for catheter coating – Dissolution kinetics and antibiofilm properties. Europ. J.of Pharma. Sci. 2018; 112. 1–7.
- Aleksandrovna P M, Aleksandrovich S O, Yurievna V S, Vladimirovich K I, Igorevich P A, Grigorievich P A. Inhibiting Effect of Electrochemically Activated Aqueous Solutions on Growth Biofilms. Int. J. Pharm. Res. Allied Sci. 2019; 8(2): 150-156.
- Pogorelov A G, Bakhir V M, Ipatova L G, Kuznetsov A L, Suvorov O A, Kozlov I V. Modeling, formation, destruction and scanning electron microscopy of biofilms. Int. J. Pharm. Res. Allied Sci. 2017; 6(1): 145-152.
- Kahiesh Esfandiari M, Ina-Salwany M Y, Aslah M, Soltani M, Sabili A, Karimi M, Muthukrishnan S. Growth Performance, Palatability and Water Stability of Oral FeedBased Vaccines Against Streptococcus Agalactiae in Red Tilapia (Oreochromis sp.). J. Biochem. Tech. 2019; Special Issue (2): 106-115.
- Shamsoddin E, Mahboobi F, Kargar K, Latifi F. Etiologic Role of Bacterial Microorganisms in Medication Related Osteonecrosis of the Jaws: A Systematic Review. Int. J. Pharm. Phytopharm. Res. 2019; 9(3): 64-72.
- Dohnt, K., Sauer, M., Müller, M., Atallah, K., Weidemann, M., Gronemeyera, P., Rasch, D., Tielen, P., Krull, R. An in vitro urinary tract catheter system to investigate biofilm development in catheter-associated urinary tract infections. J. of Microbiolo Methods, 2011; 87. 302–308
- Dimitrova, K. Nanostructured coatings for controlling bacterial biofilms and antibiotic resistance. Doctoral thesis, Universitat Politècnica de Catalunya, 2017.
- Welch, W., Cai, Y., Strømme, M. A Method for Quantitative Determination of Biofilm Viability. J. Funct. Biomater., 2012; 3, 418-431.
- Parrino, B., Schillaci, D., Carnevale, I., Giovannetti, E., Diana, D., Cirrincione, G., Cascioferro, F. Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur. J. of Medi. Chem. 2016; 161, 154-178.
- Nazlıa, M., Baygarb, T., Donmezc, D., Derea, O., Uysald, I., Aksozeke, A., Işıkf, V., Akturkc, S. Antimicrobial and antibiofilm activity of polyurethane/Hypericum perforatum extract (PHPE) .compo. Bioorga.Chem. 2016; 82, 224–228
- Veerachamy, S., Yarlagadda, J., Manivasagam, G., Yarlagadda, P. Bacterial adherence and biofilm formation on medical implants. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, 2014; 228(10), 1083-1099.
- Mermel, L.A., Allon, M., Bouza, E., Craven, D.E., Flynn, P., O'Grady, N.P., Raad, I.I., Rijnders, B.J., Sherertz, R.J., Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clinical infectious diseases, 2009; 49(1), 1-45.
- Schierholz, M., Rump, F., Pulverer, G. Clinical and preclinical efficacy of antimicrobial catheters.Anasthesiol Intensivmed Notfallmed Schmerzther. Artic in German, 1997; 32(5):298-305.
- Richard, G., Nina, C., Hibbertb, D., Kathryn, A. Antimicrobial strategies to reduce polymer biomaterial infections and their economic implications and considerations. Inter Biodeter& Biodegr 2019; 136, 1–14.
- Bardawell, K., Gallimore, B., Gagnon F., Subang R. D-amino acids: Prospects for new therapeutic agent. J. of medic and Bioeng. 2014; 3(3): p197.
- PrescottL, M., Harley, P., Klein, A. Performance Standards for Antimicrobial Disk Susceptibility Tests. L. E. in Microbi, Fifth Edition, 2002; 29(1). ISSN 0273-3099
- Yusuf, D., Begum, A., Ahsan, R. Antibiotic sensitivity pattern of gram negative uropathogenic bacilli at a private hospital in Dhaka J .Med. Sci; 8(3):189-194.
- Prescott, M., Harley, P., Klein, A. Laboratory Exercises in Microbiology, Fifth Edition .W.c.b. Mc.u. S. a, 2002.
- Juda, M., Helon, P., Malm, A. Anti-adhesive and antibiofilm activity In Vitro of Linzolid, Vancomycin, Tigecyclin, and Daptomycin against Staphylococcus haemolyticus. Acta. Polon. Pharmac. Drug Research, 2016; 73(6): 1539-1543.
- Salman, S., Al Marjani, F., Abdulrazaq, A., Salman, A, Nawar, B., Kam, B. Antibiofilm effect of iron oxide nanoparticles synthesized by lactobacillus fermentation on catheter. Wrld J. of pharmaceut. res. 2015; 4(8).
- Delcaru, C., Alexandru, I., Podgoreanu, P., Grosu, M., Stavropoulos, E., Chifiriuc, C., Lazar, V., Microbial biofilms in urinary tract infections and prostatitis: etiology, pathogenicity, and combating strategies. Pathogens. 2016;5(4):65.
- Kai, P., Jianming, D., Zhijum, G., Qin, B. Characteristics and development trends of ecohydrology in lakes and reservoirs: Insi. From bibliomet. Ecohydrol, 2019; 12(3), article number: 2080
- Lin, W., Heidi, H., Zhao, J., Han, L., Zhu, Y., Akter, J., Wickremasinghe, H., Walpola, H., Wirth, V., Rao, G., Forrest, A., Velkov, T., Lia, J. Polymyxin B in Combination with Enrofloxacin Exerts Synergistic Killing against Extensively Drug-Resistant Pseudomonas aeruginosa .J. Antimicrobial Agents and Chem. 2018; 62(6): 28-18
- Chan, L., Yee, Y., Raja, I., Ya p , Y. Short Communication Synergistic effect of non-steroidal ant inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus .J.of Glob Antimicrobial Resist. 2017; 10, 70-74.
- Sabir, N., Ikram, A., Zaman, G., Satti, L., Gardezi, A., Ahmed, A., Ahmed, P. Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance MBBS, MCPS, FCPS, FRCP. Amer. J. of Infec. Control, 2017.
- Sara, S. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. .Hinda. Publ. Corpor. Advances in Biol. Volume, Article ID 543974, 2014; 13 pages.
- Brown, L., Vliet, H., Betts, P., Reuter, M. Tetrazolium reduction allows assessment of biofilm formation by Campylobacter jejuni in a food matrix model. J. Appl. Microbiol. Nov; 2013; 115(5):1212-21.
- Perez, M., Jorge, C., Lozano, D., Nuñez, S., Tanoira, R., Conde A., Arenas, J. M. Lopez, H., Damborenea, J., Barrena, E., Esbrit, P., Esteban, J. Evaluation of bacterial adherence of clinical isolates of Staphylococcus sp. using a competitive model. 2017; vol. 6.
- Moreno, G., Trampuz, A., Luca, D. Synergistic antibiotic activity against planktonic and biofilm-embedded Streptococcus agalactiae, Streptococcus pyogenes and Streptococcus oralis. J AntimicrobChemother. 2017; 72(11):3085-3092. doi: 10.1093/jac/dkx265
- Bhandaria, B., Charles, W., Acosta-Martinezb, V., Cottonb, J., Cano, A. Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures Applied Soil Ecology. 2018; 132: 179-186.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
