The difference of Curcumin and Antioxidant activity in Curcuma Xanthorrhiza at different regions
Ali Rosidi
Nutrition Department, Muhammadiyah University, Semarang, Indonesia.
Correspondence: Ali Rosidi, Jl. Pedurungan Tengah 9D No. 6, Semarang 50192, Central Java, Indonesia. Email: [email protected].
|
ABSTRACT
Temulawak (Curcuma xanthorrhiza Roxb) is one of the plants originates from Indonesia. An active component so-called curcumin is considered as an antioxidant. This study was aimed to analyze the curcumin and antioxidant activity of temulawak extract in two different regions. Nine-month-old temulawak rhizomes originated from 2 places named Bener Purworejo, and Tembalang Semarang were applicated. The liquid-liquid extraction method was used. Curcumin level and antioxidant activity were assessed by spectrophotometry and DPPH, respectively. Collected data were analyzed by SPSS software and were presented in narrative form. The level of curcumin in temulawak extract from Bener Purworejo (34,06±0,10%) was slightly higher compared with the curcumin level in temulawak extract from Tembalang Semarang (34,02±0,10%). Furthermore, the antioxidant activity of temulawak extract from Bener Purworejo also was a little higher (91.02±3.41 ppm) compared with the antioxidant of temulawak extract from Tembalang Semarang (94.64±4.74 ppm). In conclusion, there is no different curcumin level and antioxidant activity between temulawak extract from Bener Purworejo and temulawak extract from Tembalang Semarang. Keywords: Temulawak, curcumin, antioxidant activity. |
Introduction
Medicinal plants have been applied for centuries as a cure for different human diseases.[1] Temulawak (Curcuma xanthorrhiza Roxb), that is originated from Indonesia, is considered as a traditional medicine that has a potency to cultivate due to its medicinal functions.[2] Temulawak rhizome is well-known due to its pharmacological characteristics, including antioxidant, anti-cholesterol, anti-inflammation, anti-bacterial, appetite improvement, anemia inhibitor, and anti-cancer properties.[3] One of the well-known bioactive substances in temulawak rhizome, which has beneficial features as a medicine, is curcuminoid, which is resulted from the secondary metabolism of temulawak.[4] A component of curcuminoid, which features a yellow curcumin compound, has a specific flavor with a slightly bitter taste but non-toxic. By chromatogram HPLC, the main compounds of curcuminoid such as curcumin (61-67%), desmethoxycurcumin (22-26%), bisdemethoxycurcumin (1-3%), and curcuminoid derivatives (10-11%) are identified in temulawak.[4, 5]
Curcumin, found in temulawak, is an active component that is considered as an antioxidant. Some studies showed that curcumin of temulawak rhizome has a beneficial effect as an antioxidant. According to the recent results, the roles of antioxidants as health-promoting factors are significant.[6] The antioxidant compounds have the potential to induce endogenous antioxidant defense systems.[7] Previous studies demonstrated that phenolic compounds considered as an antioxidant are found in curcuminoid.[8, 9] Moreover, the study by Nurcholis and Bintang (2017) concluded that phenolic compounds and antioxidant activity found in temulawak are better than those found in temu ireng.[10] Also, Rao (1995) found that curcumin in temulawak was more active than either vitamin E or beta carotene.[11]
Curcumin level in temulawak is associated with environmental factor, superior seedling properties, harvest-age, altitude, cultivation method, nutrient soil availability, plant protection, and postharvest management.[12-14] According to in vitro study by Andini et al. (2015), the improvement of curcumin in temulawak can be achieved by the increasing of Mo concentration.[2] Mo element is linked to nitrate reductase activity in the amino acid formation, which is a precursor of curcumin biosynthesis.[15, 16] Furthermore, according to Purwakusumah (2016), the maturity stage of temulawak rhizome is related to the rich content of curcuminoid concomitant with high antioxidant properties.[17] High quality of rhizome is found in nine-month-old temulawak rhizome. This study was conducted to compare the curcumin level and antioxidant activity of temulawak in temulawak producing areas, Bener Purworejo, and Tembalang Semarang areas.
Materials and Methods
Tools and Materials
High quality of chemical materials such as ethanol, n-hexane, methanol, curcumin standard, and DPPH were used. The maceration apparatus was used in this study including glass jar, aluminum foil, wood stirrer, Buchner funnel, vacuum pump (BIOBASE), rotary evaporator (BIOBASE), spectrophotometer UV-Vis (AMTAST), analytic balance (OHAUS), and micropipette.
Plants Materials and Sample Preparation
Nine-month-old temulawak rhizomes were purchased from Bener Purworejo and Tembalang Semarang areas. Dried temulawak rhizomes (2 kg) were mashed to yield 500 g of turmeric powder. Tumeric powder was macerated with ethanol for 2 x 2 hours and was filtered for 1 x 24 hours. The extract was then concentrated by a rotary evaporator. The extracts were purified using n-hexane by liquid-liquid extraction method with a comparison of ethanol extract: n-hexane 1:3. The liquid-liquid extraction was conducted twice for 30 minutes in each extraction. N-hexane solvent was used to dissolve non-polar compounds and fatty components of the extract. This extraction resulted in two layers; the top layer contained n-hexane phase and base layer contained ethanol phase. The separated phase between ethanol and n-hexane was due to the higher density of ethanol (ρ: 0.7893 g/ml) than n-hexane (ρ: 0,6606 g/ml). The solvent in the ethanol phase was then evaporated using rotary evaporation, which resulted in concentrated ethanol extracts. The extracts were used to quantify curcumin and antioxidant level.
Curcumin Level Analysis
100 ppm of standard curcumin was added to a 100 ml volumetric flask containing 100 ml ethanol. The solution was then diluted to 0, 2, 4, 6, 8, and 10 ppm. Curcumin standard absorbance was monitored at 425 nm. 100 mg of the sample was extracted with 5 ml of ethanol in triple repetitions. The obtained filtrated was then evaporated using nitrogen gas over the water bath, which yielded a concentrated solution. The concentrated solution was mixed with 10 ml of ethanol in a volumetric flask. Curcumin standard absorbance was monitored at 425 nm. Curcumin level was quantified by the formula:
Curcuminooid Total 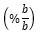
Antioxidant Activity Analysis
The determination of antioxidant activity was done using DPPH (2,2-diphenyl-1-picrylhydrazyl) and spectrophotometry method; the absorbance was measured in a wavelength of 517 nm. To determine the antioxidant activity, the extract was dissolved in 200 μl of methanol. Then, 200 μl of 0.1 M buffer acetate (pH 5.5) and 100 μl of 0.0005 M DPPH solution were added to the solution. After 30 minutes of incubation at 37 ℃, IC50 was calculated through 50% absorbance of DPPH solution.
Result and Discussion
Curcumin Level in Temulawak Extract
Temulawak used in this study was purchased from Tembalang Semarang and Bener Purworejo areas, Central Java. The main components of temulawak rhizome fraction are curcuminoid, essential oil, and starch.[3, 18] Moreover, the main compounds of curcuminoid found in temulawak are curcumin and desmethoxycurcumin.[10, 19] The percentage of curcumin in temulawak extract (Figure 1) from Bener Purworejo (34.06±0,10%) was slightly higher than curcumin in temulawak extract from Tembalang Semarang (34.02±0,10%.).
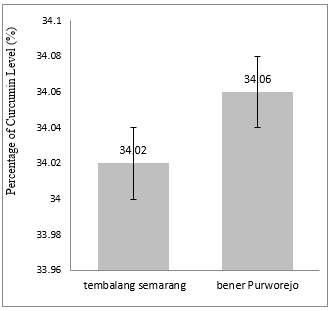
Figure 1. Curcumin Level of Temulawak Extract in a different place
The curcumin level in this study was higher than the result of the previous study carried out by Rosidi (2016), which reported that the percentage of curcumin in temulawak was 27.19%.[20] According to Wardiyati et al. (2012; quoted by Murdiono et al., 2016), numerous factors can influence the level of curcumin found in temulawak rhizomes, such as genetic and environmental factors.[21] The environmental factors including climate, sunlight, temperature, atmosphere features (CO2, O2, and humidity), physical and chemical characteristics, and water availability can affect curcumin level.[22] Furthermore, the most influencing environmental factors toward curcumin level, according to Murdiono et al., (2016) are rainfall intensity and nutrient soil availability. Temulawak plants grow and reproduce well in the annual rainfall region between 1000 and 4000 mm. The previous study by Murdiono (2016) demonstrated that temulawak rhizome production linearly correlates with the rainfall intensity.[21] Moreover, a study carried out by Andini et al., (2015) showed that the improvement of Mo in soil could decrease the growth of leaves by 39.52%, yet it increased curcumin level by 79.36%. In another study, the temperature was not an influencing factor toward both curcumin level and rhizome weight.[2] Instead of N and Mg in soil, which have negative correlation toward curcumin level found in temulawak, rhizome weight depends on P and K of soil.[23]
Extraction is the first step in the study of medicinal herbs. Crude extract preparation is the starting point to isolate and purify the chemical composition of the plant.[24] Liquid-liquid extraction method with hexane solvent (with a ratio of raw material: solvent of 1:3) was used to extract temulawak; each extraction took 30 minutes. The principle of liquid-liquid extraction is based on solvent distribution with a specific ratio of separated solvent (Khopkar 1990). In the extraction method, the different solvent systems become determinant factor depending on the main compound of temulawak rhizome, which is rich in antioxidants. The solvent used should attract the active component and should not be mixed with either solid or liquid compound. By intensive contact, the active component of the compound can migrate to solvent.[10, 25] Differentiation of curcumin level is affected not only by extraction method but also by ripening stage of temulawak when it was harvested. A study by Rosiyani (2010; quoted by Rosidi, 2016) demonstrated that the highest curcumin level is in nine-month-old temulawak rhizome.[20]
Antioxidant Activity of Temulawak Extract
Antioxidant activity in this study was assessed by 1,1-diphenyl-2picrylhydrazil (DPPH), which is well-known as a simple, fast, and sensitive method. Antioxidant activity assessment by DPPH method shows the ability of antioxidant in general, not including the specific radicals inhibited.[26, 27]
This study elucidated that the antioxidant activity of temulawak originated from Bener Purworejo (91.02±3.41 ppm) was better than the antioxidant activity of temulawak originated from Tembalang Semarang (94.64±4.74 ppm) (Figure 2). IC50 has defined the concentration which inhibits 50% free radical activity of DPPH. The lower IC50 value indicates better antioxidant activity.[28, 29] The variety of antioxidant activity values are affected by the difference of secondary metabolite compounds found in temulawak rhizome in various regions.[17] Furthermore, the soil nutrient differences and local variables have a role toward secondary metabolite biosynthesis. The main curcuminoid compounds found in temulawak is curcumin and desmethoxycurcumin. According to Molyneux (2004), the antioxidant activity of temulawak extract originated from Bener Purworejo and Tembalang Semarang have a strong antioxidant activity (50-100 ppm).[29] Besides, the substance is categorized as an active antioxidant if it possesses IC50 value at a range of 50 to 100 ppm. [30]
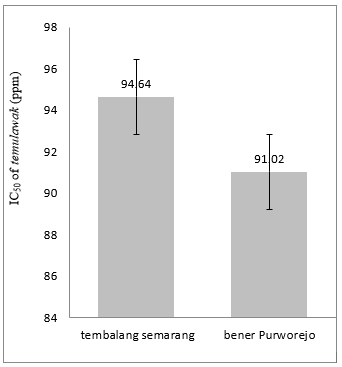
Figure 2. IC50 of temulawak Extract from a different place
Antioxidant activity assessed by the DPPH method is affected by the active compounds of temulawak extract. The active compounds of temulawak played some roles as oxidants and radicals. The reactive groups of DPPH contain nitrogen groups and are paired to hydrogen atom thereby generating stable radical DPPH. The ability of an antioxidant to absorb radical DPPH can be seen by the change of color. The mechanism of color intensity reduction is through single electron transfer leading to the change of color from purple to yellow. The electron donor affects the color degradation from purple to brownish-yellow indicating high antioxidant concentration in the extract. Antioxidant activity assessed by the DPPH method is based on radical DPPH absorption by the antioxidant compound. DPPH is a stable free radical either in aqueous solution or in methanol solution, and it has strong absorbency at a wavelength of 517 nm.
According to the curcumin level and antioxidant activity seen in figures 1 and 2, the level of curcumin might have a relation with the antioxidant activity which is the higher curcumin level, the stronger antioxidant activity. This study was in agreement with the results of Purwakusumah et al. (2016) which demonstrated that there is a positive correlation between the active metabolite number and antioxidant activity.[17] The substitution of methoxy groups in curcumin by hydrogen has a role to scavenge radicals when antioxidant activity was assessed by DPPH.
Conclusion
Temulawak extract originated from Bener Purworejo and Tembalang Semarang have a similar curcumin level and antioxidant activity. Further research should be carried out in a wider area of Central Java so that the generalizability of findings can be disseminated through the area on the benefit of this abundant local plant.
Acknowledgments
The authors would like to express thanks to the management of the district head of Bener Purworejo and Tembalang Semarang. This article’s publication was supported by Muhammadiyah University, Semarang, Indonesia.
References
- Saida K, Sofiane K, Amel B. Phytochemical, Free Radical Scavenging and Antimicrobial Activities of the Maize Stigmas, Collected of Ain Mlila (East Algeria). World Journal of Environmental Biosciences. 2018;7(4):35-40.
- Andini IM, Roviq M, Nihayati E. The Growth and Curcumin Level of Temulawak (Curcuma xanthorrhiza Robx.) with Micronutrient Soil Availability (Mo) in In Vitro. Plant Prod J. 2015;3.
- Kawiji, Atmaka W, Oktaviana PR. Study on Antioxidant Activity, Total Phenol, and Concentration Curcuminoids Extract Curcuma (Curcuma xanthorrhiza Roxb) in Various Drying Technique and The Proportion of Dissolution. J Teknol Has Pertan. 2011;4(2):84–9
- Cahyono B, Diah M, Huda K. The Effect of Temulawak (Curcuma xanthorrhiza Roxb) Rhizome Drying toward Curcuminoid Level and Composition. Reaktor. 2011;13(3):165–71.
- Stankovic I. Chemical and Technical Assessment 61st JECFA. Assessment. 2004;1(8):1–8.
- Jamshidi S, Beigrezaei S, Faraji H. A Review of Probable Effects of Antioxidants on DNA Damage. International Journal of Pharmaceutical and Phytopharmacological Research (eIJPPR). 2018 Oct 1;8(5):72-9.
- Alshubaily FA, Jambi EJ. The Possible Protective Effect of Sage (Salvia Officinalis L.) Water Extract Against Testes and Heart Tissue Damages of Hypercholesterolemic Rats. International Journal of Pharmaceutical and Phytopharmacological Research. 2018 Feb 1;8(1):62-8.
- Bos R, Windono T, Woerdenbag HJ, Boersma YL, Koulman A, Kayser O. HPLC‐photodiode array detection analysis of curcuminoids in Curcuma species indigenous to Indonesia. Phytochemical Analysis: An International Journal of Plant Chemical and Biochemical Techniques. 2007 Mar;18(2):118-22.
- Lechtenberg M, Quandt B, Nahrstedt A. Quantitative determination of curcuminoids in Curcuma rhizomes and rapid differentiation of Curcuma domestica Val. and Curcuma xanthorrhiza Roxb. by capillary electrophoresis. Phytochemical Analysis: An International Journal of Plant Chemical and Biochemical Techniques. 2004 May;15(3):152-8.
- Nurcholis W, Purwakusumah ED, Rahardjo M, Darusman LK. Variation of Bioactive Compound and Bioactivities of Three Temulawak Promising Lines at Different Geographical Conditions. J. Agron. Indonesia.2012;40(2):153-159.
- Inayatilah FR. The Effects of Curcuma Rhizome Extract (Curcuma xanthorrhiza Roxb.) Treatment Using Various Doses Towards Endometrial Thickness in Mice (Mus musculus) Treated with Monosodium Glutamate (MSG). Journal of Islamic Pharmacy. 2017 Apr 1;2(1):38-44.
- Sahoo N, Manchikanti P, Dey S. Herbal drugs: standards and regulation. Fitoterapia. 2010 Sep 1;81(6):462-71. Available from: http://dx.doi.org/10.1016/j.fitote.2010.02.001
- Alaerts G, Dejaegher B, Smeyers-Verbeke J, Vander Heyden Y. Recent developments in chromatographic fingerprints from herbal products: set-up and data analysis. Combinatorial chemistry & high throughput screening. 2010 Dec 1;13(10):900-22.
- Rahardjo M. The Implementation of Procedure Operational Standard of Ginger as Potential Medical Raw Material. Perspektif. 2010;9(2):78–93.
- Lohry R. Micronutrients : Functions, Sources, and Application Methods. Indiana CCA Conf Proc. 2007;(Cl):
- Marschner H. Marschner’s Mineral Nutrition of Higher Plants. 3rd Editio. Germany; 2011. 1–672 p.
- Purwakusumah ED, Royani L, Rafi M. The Evaluation of Antioxidant Activity and Major Secondary Change Metabolite of Ginger (Curcuma xanthorriza) in Various Age of Rhizome. J Jamu Indones. 2018;1(1):10–7.
- Djamhari S. The Breaking of Temulawak (Curcuma Xanthorrhiza Roxb) Rhizome Dormancy with Atonic Solution and Rooting Stimulation by Auxin Application. J Sains dan Teknol Indones. 2010;12(1):66–70.
- Hsu CH, Cheng AL. Clinical studies with curcumin. InThe Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease 2007 (pp. 471-480). Springer, Boston, MA.
- Rosidi A, Khomsan A, Setiawan B, Riyadi H, Briawan D. Antioxidant Potential of Temulawak (Curcuma xanthorrhiza roxb). Pakistan Journal of Nutrition. 2016 Jun 1;15(6):556-60.
- Murdiono WE, Azizah N, Nihayati E. The Growth Response of Ginger (Curcuma xanthorrhiza Roxb.) in Fertilizer Addition and in The Dry Season. Natl Prociding PERHORTI 2014:527–32.
- Huntingford C, Hugo Lambert F, Gash JH, Taylor CM, Challinor AJ. Aspects of climate change prediction relevant to crop productivity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005 Oct 24;360(1463):1999-2009.
- Wardiyati T, Rinanto Y, Sunarni T, Azizah N. Collection and Identification of Curcuma Zzanthoriza And Curcuma Domestica In Java and Madura Islands: 2. Genetic Diversity. J Ilmu-Ilmu Hayati. 2009;21:50-59.
- Vivekananda M, Yogesh M, Hemalatha S. Microwave Assisted Extraction - An Innovative and Promising Extraction Tool for Medicinal PHCOG REV.: Review Article Extraction Tool for Medicinal Plant Research. Pharmacogn Rev. 2007;1(1):1–18.
- Gamse T. Estraction [Internet]. 2002. Available from: papers3://publication/uuid/3D5959B9-3CDC-4113-99CF-E968E829C731
- Juniarti, Osmeli D, Bagian Y. Chemical Content, Toxicity Assay (Brine Shrimp Lethality Test) and Antioxidant (1,1-diphenyl-2-pikrilhydrazyl) of Jequirity Bean Leaves Extarct (Abrus precatorius L.). Makara Sains. 2009;13(1):50–4.
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. African journal of biotechnology. 2006;5(11).
- Hanani E, Mun’im A, Sekarini R. The Antioxidant Identification in Callyspongia SP from Kepulauan Seribu. Ilmu Kefarmasian. 2005;2(3):127–33.
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004 Mar;26(2):211-9.
- Jun M, Fu HY, Hong J, Wan X, Yang CS, Ho CT. Comparison of antioxidant activities of isoflavones from kudzu root (Pueraria lobata Ohwi). Journal of food science. 2003 Aug;68(6):2117-22.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
