Prevalence of Mycoplasma infection in poultry (Gallus gallus domesticus) and evaluation of some diagnostic techniques
Sabah M. Salih1*, Nihad A. Jafar2, Bashar Sadeq Noomi2
1 Clinical Lab., Sciences Dept., College of Pharmacy, University of Kirkuk, Iraq. 2 Microbiology Dept., College of Veterinary Medicine, University of Tikrit, Iraq.
ABSTRACT
Investigation for most feasible, easy, and cheapest laboratory methods for diagnosis of mycoplasma infection is a worldwide need. The present study aimed to estimate the prevalence of Mycoplasma infection (Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS)) in poultries and evaluating three serological techniques, serum plate agglutination (SPA) technique, enzyme-linked immunosorbent assay (ELISA), and Hemagglutination inhibition (HI) technique with polymerase chain reaction (PCR) technique done in previous work. Serum samples (276) were collected from chickens with respiratory and articular diseases (202 and 74 respectively). A high detecting rate for MG (31.8%) and MS (19.5%) was observed using the SPA technique in comparison with other techniques. The sensitivity and specificity of the three techniques according to the PCR technique varied, 100% specificity recorded using ELISA and HI techniques. The current study concluded that the SPA technique is a more appropriate technique for detecting MG and MS. ELISA and HI techniques are highly specific. Although, a certain level of false-positive results can be expected in any test.
Keywords: Mycoplasma, poultry, SPA, ELISA, Hemagglutination Inhibition
Introduction
Mycoplasma spp. are associated with different diseases [1-3]. The most important poultry mycoplasmosis is caused by Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS) [4]. The diseases caused by these microorganisms include chronic respiratory disease of chicken caused by MG and infectious synovitis of chicken caused by MS [5]. Symptoms of respiratory infection include respiratory rates, coughing, and nasal discharges. Infection with MS, on the other hand, causes acute or chronic disease of chickens and turkeys constitutes primarily an infection of synovial membranes of joints and tendons sheath, also, it causes airsacculitis [5].
The techniques used in the detection of mycoplasma infection are very important since clinical diagnosis (signs and pathological lesions) cannot confirm the source of infection. Prevention of the spread of infection and decreasing the economic losses in the poultry products are an attractive vision especially in the finding of the most reliable and rapid diagnostic detection of mycoplasma infections. Three approaches in the diagnosis of mycoplasma infection were submitted, they are, isolation and identification of the microorganisms via culture method, detecting of its DNA, and identification of specific antibodies in the serum [4, 6, 7]. The serological tests which are commonly used for the diagnosis of mycoplasma include serum plate agglutination (SPA), enzyme-linked immunosorbent assay (ELISA), and hemagglutination inhibition (HI) techniques [8-16].
The present study aimed to estimate the rate of mycoplasma infection in chickens with respiratory and articular diseases and evaluation of some serological diagnostic methods.
Material and Methods
Samples collection:
Blood samples were collected from 276 infected chickens suffering from respiratory disease including (cough, sneeze, nasal discharge, and inflammation of eyes) and/or joint infection (lameness and swelling of joints).
Serological tests:
Serum plate agglutination (SPA) test: Fresh sera were tested against MG and MS antigens (Charles River-USA), following the manufacturer's instructions. Briefly, thirty µl of serum was mixed with thirty µl of antigen and then incubated at room temperature (25 ºC) for 1-2 min before the result was read. Negative and positive sera were included in each test.
ELISA test: It was applied by using 2 kits (Biochek company-Holland); one for diagnosis of MS and the other for MG.
Hemagglutination inhibition (HI) test: The test was done in the Microtiter plate using kits provided by Charles River-USA. The kit contained two types of antigens for the bacteria MG and MS, and the test was done according to [17].
Evaluation of the test techniques:
The results of the 3 serological tests were evaluated according to the results of PCR for the same samples which were recorded in the previous paper [18].
Statistical analysis:
- Positive agreement =No. of samples gave positive results in the first test No. of samples gave positive results in the second test ×100

- Negative agreement = No. of samples gave negative results in the first testNo. of the samples gave negative results in the second test ×100
 [16].
[16].
- Sensitivity=True positiveTrue positive+false negative ×100
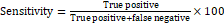
- Specificity= True negative True negative+ false positive ×100
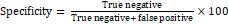
- Positive predictive values = True positiveTrue positive + false positive ×100

- Negative predictive values = True negative True negative + false negative ×100
 [17].
[17].
Results:
In the current study, chickens suffering from the two types of diseases were studied.
First group: Chickens with respiratory signs including cough, sneeze, nasal discharge, and inflammation of eyes.
Second group: Chickens with lameness and joint swellings.
Serological tests:
Serum plate agglutination test (SPA): The results showed that out of 276 sera samples, a total of 142 (51.4%) gave positive anti-MG and anti-MS. Infection with MG [88(31.8%)] was more than MS infection [54 (19.5%)]. In addition, respiratory MG infection [84(41.5%)] was more than MS infection [27(13.3%)]. While MS infection [27(36.4%)] was more than MG infection [4(5.4%)] in chickens with joint diseases (Table 1).
|
Table 1: Serum plate agglutination test for chickens suffering from respiratory and articular sings. |
|||||||
|
Type of samples |
No of samples |
Positive samples for MG antibody |
Positive samples for MS antibody |
Total |
|||
|
No |
% |
No |
% |
No |
% |
||
|
Respiratory diseases |
202 |
84 |
41.5% |
27 |
13.3 |
111 |
54.9% |
|
Articular diseases |
74 |
4 |
5.4% |
27 |
36.4% |
29 |
39.1% |
|
Total |
276 |
88 |
31.8% |
54 |
19.5% |
142 |
51.4% |
When comparing the SPA test and PCR for MG & MS, the results of sensitivity and specificity were respectively 80.9% and 76.9% for MG and 91.7% and 90.4% for MS (Tables 2&3).
|
Table 2: Comparison between PCR and serum plate agglutination (SPA) test for MG. |
|||||
|
|
Result of SPA test |
Total |
|||
|
Positive |
Negative |
||||
|
Result of PCR |
True positive |
34 |
False-positive |
54 |
88 |
|
False-negative |
8 |
True negative |
180 |
188 |
|
|
Total |
|
42 |
|
234 |
276 |
Sensitivity = 80.9% Specificity = 76.9%
|
Table 3: Comparison between PCR and serum plate agglutination (SPA) test for MS. |
|||||
|
Result of SPA test |
Total |
||||
|
Positive |
Negative |
||||
|
Result of PCR |
True positive |
31 |
False-positive |
23 |
54 |
|
False-negative |
3 |
True negative |
219 |
222 |
|
|
Total |
|
34 |
|
242 |
276 |
Sensitivity = 91.7% Specificity = 90.4%
Results of ELISA test: ELISA test detected antibodies against MG in 43 (21.1%) from the total of 202 chickens with respiratory sings, in contrast, anti-MS was detected in only 9 (4.4%) of the same chickens. In cases of chickens with articular sings, ELISA test detected antibodies against MS in 17 (22.9%) of the total 74 chickens, while, it detected antibodies against MG in only 5 (6.7%) from the same chickens. This means that the ELISA technique detected antibodies against MG in 48 (15.7%) chickens with respiratory or articular signs and detected antibodies against MS in 26 (9.4%) chickens (Table 4).
|
Table 4: ELISA test results for anti-MG and anti-MS in chickens with respiratory and articular signs. |
|||||||
|
Type of disease |
No. of samples |
Positive samples for MG antibody |
Positive samples for MS antibody |
Total |
|||
|
No |
% |
No |
% |
No |
% |
||
|
Respiratory signs |
202 |
43 |
21.2% |
9 |
4.4% |
52 |
25.7% |
|
Articular signs |
74 |
5 |
6.7% |
17 |
22.9% |
22 |
29.7% |
|
Total |
276 |
48 |
15.7% |
26 |
9.4% |
74 |
26.8% |
When comparing ELISA test results with PCR, the sensitivity and specificity of the ELISA test was 73.8% and 92.7% for MG and 76.4% and 100% for MS (tables 5 & 6).
|
Table 5: Sensitivity and specificity of ELISA test according to PCR in the diagnosis of MG. |
|||||
|
Result of PCR |
Result of plate agglutination test |
Total |
|||
|
Positive |
Negative |
||||
|
True positive |
31 |
False positive |
17 |
48 |
|
|
False negative |
11 |
True negative |
217 |
228 |
|
|
Total |
|
42 |
|
234 |
276 |
sensitivity= 73.8% specificity= 92.7%
|
Table 6: Sensitivity and specificity of ELISA test according to PCR in the diagnosis of MS. |
|||||
|
Result of PCR |
Result of Serum plate agglutination test |
Total |
|||
|
Positive |
Negative |
||||
|
True positive |
26 |
False positive |
0 |
26 |
|
|
False negative |
8 |
True negative |
232 |
240 |
|
|
Total |
|
34 |
|
232 |
276 |
sensitivity= 76.4% specificity= 100%
- Positive agreement between ELISA and Serum plate agglutination test for MG and MS was 54.5% and 48.1% respectively.
- Total agreement between ELISA and Serum plate agglutination test for MG and MS was 49.4% and 25.3% respectively.
Results of Hemagglutination inhibition test (HI):
HI test for MG gave positive results at a rate of 12.3%, while the test for MS gave positive results at a rate of 9.7% (Table 7).
|
Table 7: Results of Hemagglutination inhibition test. |
|||||||
|
Type of samples |
No of samples |
Positive samples for MG antibody |
Positive samples for MS antibody |
Total |
|||
|
No |
% |
No |
% |
No |
% |
||
|
Respiratory signs |
202 |
34 |
16.8% |
6 |
2.9% |
40 |
19.8% |
|
Articular signs |
74 |
0 |
0% |
21 |
28.3% |
21 |
28.3% |
|
Total |
276 |
34 |
12.3% |
27 |
9.7% |
61 |
22.1% |
When comparing HI test results with PCR, the sensitivity and specificity of the HI test were respectively 80.9% and 100% for MG and 79.4% and 100% for MS (tables 8 & 9)
|
Table 8: Sensitivity and specificity of HI test according to PCR in the diagnosis of MG. |
|||||
|
Result of PCR |
Result of the HI test |
Total |
|||
|
Positive |
Negative |
||||
|
True positive |
34 |
False positive |
0 |
34 |
|
|
False negative |
8 |
True negative |
234 |
242 |
|
|
Total |
|
42 |
|
234 |
276 |
sensitivity= 80.9% specificity= 100%
|
Table 9: Sensitivity and specificity of HI test according to PCR in the diagnosis of MS. |
|||||
|
Result of PCR |
Result of Serum plate agglutination test |
Total |
|||
|
Positive |
Negative |
||||
|
True positive |
27 |
False positive |
0 |
27 |
|
|
False negative |
7 |
True negative |
242 |
249 |
|
|
Total |
|
34 |
|
242 |
276 |
sensitivity= 79.4% specificity= 100%
- Positive agreement between ELISA and HI test for MG and MS: 70.8% and 96.2%, respectively.
- Total agreement between ELISA and HI test for MG and MS: 70.8% and 50.9%%, respectively.
- Positive agreement between Serum plate agglutination test and HI test for MG and MS: 38.6% and 50%, respectively.
- Total agreement between HI and Serum plate agglutination test for MG and MS: 27.8% and 33.3%, respectively.
Discussion
Various techniques can be applied in the diagnosis of mycoplasma. Serological tests have been used to detect antibodies against the pathogen, including SPA, HI, and ELISA tests. Also, tests have been used to detect the mycoplasma to find either the organisms by culture and isolation or their DNA using PCR procedures [19]. In the current study, the serological tests were performed according to the results of the PCR technique.
The results of the serological test revealed that the results of the ELISA test were similar to those of HI, since, both of them can detect IgG antibodies [20]. The results also showed that the highest positive results were recorded by the SPA test in comparison with ELISA and HI tests due to its ability in the detection of IgM. Therefore, the SPA test considered the best serological method and it is easy, cheap, and highly sensitive [20]. Even though, false-positive results can be expected in certain levels in any test [21]. So, because of the variety of false-positive results between several serologic tests, it is not advisable to depend completely on one technique [22]. The high number of false-positive results in several tests may be due to factors such as serum of avian which recently infected with a heterologous Mycoplasma spp., heat inactivation lack, age of the avian and applying inactivated vaccines [23]. The World Organization for Animal Health (OIE) recommends the use of serological techniques for avian mycoplasmosis only as screening tools in the diagnosis of flocks, not of individual birds [4, 22].
Conclusion
Respiratory M. gallisepticum (MG) infection is more frequent than M. synoviae (MS) infection in chickens. While, the MS infection is more frequent than MG infection in chickens with joint diseases. The most appropriate technique for detecting (MG) and (MS) is SPA. ELISA and HI techniques are highly specific. Although, certain level of false positive results can be expected in any test.
References
- Lestari S W, Japari A, Makes D, Wasian G, Hartono J, Supardi P, Hestiantoro A. Imaging of the Male Genital Tract: A Review of the Mechanism of Sperm Quality Impairment in Infertility. Int. J. Pharm. Phytopharm. Res. 2020; 10(1): 87-96.
- Amer M M, Zohair G A M, EL-Shemy A, Mekky H M, Sedeek D M. Immune status and reproduction of medication vaccinated broiler breeder chickens. Int. J. Pharm. Phytopharm. Res. 2018; 7(4): 18-25.
- Ahmadi-Salmasi B, Amiri-Nikpour M R, Faraj Pourvand S. Investigating The Correlation Between Level Of Igg Antibodies Against H.Pylori And Acute Non-Cardioembolic Ischemic Stroke In Adults. Pharmacophores. 2017; 8(1): 25-30.
- OIE. Avian mycoplasmosis (Mycoplasma gallisepticum, Mycoplasma synoviae) In: Manual of diagnostic tests and vaccines for terrestrial animals, 2008, 525-541.
- Khalifa KA, Abdelrahim ES, Badwi M and Mohamed AM. Isolation and molecular characterisation of Mycoplasma gallisepticum and Mycoplasma synoviae in chickens in Sudan. Journal of Veterinary Medicine: Article ID 208026, doi:10.1155/2013/208026.
- Dufour-Zavala L, Swayne DE, Glisson JR, Pearson JE, Reed WM, Jackwood MW., Woolcock PR. A laboratory manual for the isolation, identification and characterisation of avian pathogens. 5th eds. American Association of Avian Pathologists, Athens, GA. 2008: 59-64.
- Qasem JA, Al-Mouqati SA, Al-Ali EM., Ben-Haji A. Application of molecular and serological methods for rapid detection of Mycoplasma gallisepticum Infection (Avian mycoplasmosis). Pakistan Journal of Biological Sciences, 2015; 18: 81-8
- Chirinos B Z, Icochea E D, Cesar CC, No´e NM. Evaluacion de la prueba de inhibici ´ on de la hemaglutinaci ´ on ´ vs. ELISA para la deteccion de anticuerpos contra ´ Mycoplasma gallisepticum y Mycoplasma synoviae,” Revista de Investigaciones Veterinarias del Peru´, 2000; 11(1): 40–44
- Ewing ML, Kleven SH., Brown MB. Comparison of enzyme-linked immunosorbent assay and hemagglutination inhibition for detection of antibody to Mycoplasma gallisepticum in commercial broiler, fair and exhibition, and experimentally infected birds, Avian Diseases, 1996; 40(1): 13–22.
- Ewing ML, Cookson KC, Phillips RA. Turner KR, Kleven SH. Experimental infection and transmissibility of Mycoplasma synoviae with delayed serologic response in chickens. Avian Diseases, 1998; 42(2): 230–238.
- Fiorentin L, Mores MAZ, Trevisol IM et al., Test profiles of broiler breeder flocks housed in farms with endemic Mycoplasma synoviae infection. Brazilian Journal of Poultry Science, 2003; 5(1): 37–43.
- Fritz BA, Thomas CB, Yuill TM. Serological and microbial survey of Mycoplasma gallisepticum in wild turkeys (Meleagris gallopavo) from six western states. Journal of Wildlife Diseases, 1992; 28(1): 10–20.
- Kleven SH, Rowland GN, Kumar MC. Poor serologic response to upper respiratory infection with Mycoplasma synoviae in Turkeys. Avian Diseases, 2001; 45(3): 719–723.
- Noormohammadi AH, Markham PF, Markham JF, Whithear KG, Browning GF. Mycoplasma synoviae surface protein MSPB as a recombinant antigen in an indirect ELISA. Microbiology, 1999; 145(8): 2087–2094.
- Ortiz A., Kleven SH. Serological detection of Mycoplasma synoviae infection in turkeys. Avian Diseases, 1992; 36(3): 749–752.
- Swayne DE., Kleven SH. Mycoplasmosis. in A Laboratory Manual for the Isolation and Identification of Avian Pathogens, J. R. Glisson, M. W. Jackwood, J. E. Pearson, and W. M. Reed, Eds., 1988; 74–80, Kennett Square, Pa, USA.
- Jafar NA., Noomi BS. Detection of Mycoplasma gallisepticum and Mycoplasma synovise by using of cultural and PCR technique. Iraqi Journal of Veterinary Sciences. 2019; 33 (2): 469-473.
- Allan WH., Gough RE. A standard haemagglutination inhibition test for Newcastle disease.(1). A comparison of macro and micro methods. Veterinary Record, 1974; 95(6), 120-123.
- Ley DH. Mycoplasma gallisepticum infection. In: Diseases of Poultry. 11th ed. Y.M. Saif, H.J. Barnes, A.M. Fadly, J.R. Glisson, L.R. McDougald, and D.E. Swayne (eds.). Ames: Iowa State University Press, 2003; 122 - 144.
- Kleven SH, Fan HH, Turner KS. Pen trial studies on the use of live vaccines to displace virulent Mycoplasma gallisepticum in chickens. Avian diseases, 1998; 300-306.
- Feizi A, khakpour M, Nikpiran H, kaboli k, Bijanzad P, Moggadam ARJ, Hosseini H. Comparative Evaluation of Serological test in Diagnosis of Mycoplasma gallisepticum Infection in Iranian North-west rural Poultry. Adv. Biores. 2013; 4(3): 50-53
- Feberwee A, Mekkes DR, De Wit JJ, Hartman EG. Pijpers A. Comparison of culture, PCR, and different serologic tests for detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections. Avian Dis. 2005; 49(2): 260-8.
- Ross T, Slavik M, Bayyari G., Skeeles J. Elimination of mycoplasmal plate agglutination cross-reactions in sera from chickens inoculated with infectious bursal disease viruses. Avian Dis. 1990; 34(3): 663-7.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
