Influence of Raphanussativus seeds on sperm parameters of domestic rabbit (Oryctolaguscuniculus) in cadmium-induced toxicity
Moumen Yasmina1*, Abdennour Cherif2, Benbott Amel1, Djemil Randa3, Khiel Saida1, Hamadouche Nadira1
1 Laboratory of Biomolecules and Plant Breeding, Department of Life Sciences and Nature, Faculty of Exact Sciences, Life Sciences and Nature, University of Larbi Ben M’hidi, Oum El-Bouaghi 04000, Algeria.2Laboratory of Animal Ecophysiology, Department of Biology, Faculty of Sciences, University ofBadjiMokhtar-Annaba, Annaba 23000, Algebria.3 Department of Biology, University of Khenchela 44000, Algeria.
Correspondence: MoumenYasmina, Laboratory of Biomolecules and Plant Breeding, Department of Life Sciences and Nature, Faculty of Exact Sciences, Life Sciences and Nature, University of Larbi Ben M’hidi, Oum El-Bouaghi 04000, Algeria. Email: moumenyasmina @ hotmail.fr
|
ABSTRACT
The present study was conducted to determine the possible protective effect of ethanol extract of radish (Raphanussativus; Rs) seeds on reproductive markers of local male rabbits (Oryctolaguscuniculus) intoxicate by cadmium (Cd). Twenty adult rabbits were divided into equally four groups; the control, the CdSO4 (0.15 mg/kg bw), the Rs (15 mg/kg bw) and the combined group (Cd-Rs). The Cd and the Rs were administrated daily by gavage for a period of four weeks, and then the reproductive organs’ weight, the plasma testosterone, and the biology of sperms were evaluated. The results showed the toxic effect of Cd manifested by the significant decrease of the relative weights of reproductive organs (testis and epididymis) and plasma testosterone level, accompanied with the spermatozoa concentration, speed and motility. However, in the Rs positive group, a remarkable rise in the biology of spermatozoa, the organs’ relative weights, and the testosterone concentration were recorded. On the other hand, the previous markers in the Cd-Rs group were almost identical to the control, except the spermatozoa concentration which was significantly higher, demonstrating the beneficial use of the extract. The histological profiles revealed some changes in the spermatogenesis and the organs’ architecture such as the absence of spermatid phase, the narrowing of seminiferous walls, and the increase in the interstitial space of Cd-treated rabbits. Contrary, the histology of organs showed less deteriorations in the Cd-Rs group, indicating the preventive activity of the radish seed ethanol extract towards cadmium induced toxicity.
Keywords:CdSO4, male rabbit, radish seeds, reproduction, sperm biology, toxicity. |
Introduction
Since the 19th century, the world has experienced important developments in the industrial, agricultural and urban areas, accompanied by an inevitable pollution of the environment, leading to a negative effect on human health, and sometimes threatening his life. This anthropogenic pollution caused the contamination of the atmosphere and the continental waters, causing an abnormal modification of chemical levels within the environment, as the case of trace metals.
Cadmium is one of the toxic elements being utilized in different human purposes. However, it disperses easily in the environment and then enters human body mainly through ingestion of contaminated food and drinking water, inhalation of particulates from ambient air or exposure to tobacco smoke. [1] Cadmium might accumulate in many tissues such as kidney, liver, brain, muscles, testes with long biological half-life. [2, 3] producing certain disturbances of organ’s functions as that of lungs, liver, and testes. [4, 5] Reproduction was proved to be susceptible to cadmium intoxication in both rats and rabbits [6-8], in addition to humans. [9] It causes a decrease in the biosynthesis of mRNA LH receptor, testicular necrosis, reduction in sperm motility rate and derangement in spermatogenesis and spermiogenesis. [10] Steroid hormones are very important and play essential roles in maintaining reproductive functions. [11, 12]
The exposure to cadmium causes an unbalance of antioxidants, because it generates reactive oxygen species (ROS), especially during chronic intoxication. As a result, cell molecules including DNA, lipids, proteins, and enzymes including the antioxidant enzymes might be affected, leading to functional disorders [13, 14], which are considered as the basis of free radicals’ intoxication.
Currently, growing interest in natural antioxidants has been developed due to the need for more effective, less toxic and low cost antioxidants, in which the plants appear to have such desired advantages. [15] For preventing animals from cadmium toxicity, garlic bulb [16-19], sesame seeds [20], black cumin seeds [21], thyme [22], vitamin C, Curcumin [23], and resveratrol [24] have been utilized.
Radish (Raphanussativus)components have been used for their beneficial properties to human health. It is an important source of antioxidants that protect human body against many diseases, including cancer, diabetes, cholestasis and infertility. Radish has also the ability to enhance the antioxidant defense mechanism and reduce the accumulation of free radicals. [25] Radish containsanthocyanins, potent antioxidant flavonoids that possess anti-inflammatory and anti-carcinogenic activity, cardiovascular disease prevention, obesity control, and diabetes alleviation properties, all of which are more or less associated with their potent antioxidant properties. [26] Catechin was found to be the most abundant phenolic compound in radish, while sinapic acid was predominant in the methanolic and hexane extracts. Thus, the methanolic extract exhibited moderate metal chelating activity, strong ferric reducing ability, and strong free-radical scavenging activity. [25]
The aim of this study was to determine the toxic effect of cadmium on certain reproductive markers, as well as, to evaluate the possible protective activity of radish (Raphanussativus) seed extract on male adult rabbits (Oryctolaguscuniculus).
Materials and Methods
Extraction process
Radish seeds were obtained from a local herbalist in the region of Oum El-Bouaghi. The extraction of radish seeds was carried out by grinding to obtain a very fine powder, which was diluted in 70% ethanol, filtered twice, extracted by using the rotary evaporator (BUCHI) at 60 °C, and finally dried in a dark airy place for 15 days.
Experimental animals and treatment
Sixteen local male rabbits, aged 5 months old, with a mean weight of 2427.5±187.04 g was obtained from a rearing center. Local rabbit is characterized by its small size, good fertility and the ability to reproduce throughout the year in the most favorable living environment of North Algeria. [27] Animals were placed in the animal rearing house at the Department of Biology, University of Oum El-Bouaghi under standard conditions of temperature, humidity and photoperiod, in which food and water were given ad libitum.
Rabbits were then divided into four equal groups; the control, the cadmium group (Cd) treated with 0.15 mg CdSO4/kg b.w., the Raphanussativus group (Rs) supplemented with 15 mg/kg b.w. and the combined treatment group (Cd-Rs) received both 0.15 mg CdSO4/kg b.w. and 15 mg/kg b.w. cadmium and radish extract were daily diluted in 5 ml distilled water, and then administrated by gavage in the morning and in the afternoon.
Samples’ collection
After 30 days of daily treatment, rabbits were sacrificed; the blood samples were collected in heparinized test tubes, and then centrifuged at 4000 rpm for 15 minutes to obtain the plasma in order to estimate the testosterone. At the same time, the testis and the epididymis were removed and their relative weights were estimated. The testis was preserved in diluted formalin (10%) for the histological study.
Testosterone
The plasma testosterone level was assayed using the commercial kit (ST AIA-PACK Testosterone enzyme immunoassay) and the apparatus TOSOH AIA System Analizers (3-8-2 Shiba, Minato-ku, Tokyo, Japan). The concentration of testosterone was expressed as ng/ml.
Sperm parameters
To study the sperm biology, a small incision was made in the caudal epididymis and a drop of sperm was taken and diluted in 1 ml of physiological solution (NaCl 0.9%). The evaluation of the concentration, the speed and the mobility of spermatozoa was carried out according to the method of the world health organization. [28]
Histological preparation of the testis
To prepare a histological section, the testis was fixed in the diluted formaldehyde, dehydrated to remove water from the organ by using alcohol baths of increasing concentration, followed by the xylene. The blocks were carried out in special molds (bars of leucart), the sections of 4-5 μm were made by the microtome, and then placed on a slide where a drop of gelatinized water was deposited. The staining occurred after the paraffin was removed with xylene. The sample was placed in hematoxylin, followed by the eosin for 3 to 5 minutes for each. The setting was prepared by depositing a drop of Eukitt on which a cover slip was placed. After drying, the slides are ready for observation by microscope.
Data analysis
The results obtained were expressed as a mean ±SD, the statistical analysis of the data was performed using Student's t-test for the comparison of two means, whereas, the multiple comparison of the means was made by one-way analysis of variance (ANOVA). The statistical test was considered significant at p≤0.05 level.
Results
Organs’ weights
The obtained results show a significant decrease in the relative weight of the testis and the epididymis of the cadmium-treated group, whereas the group supplemented with the radish seed extract (Rs) indicates an increase in the relative weight of testis and epididymis compared to the control. However, the results of the combined treatment (Cd-Rs) reveal a significant rise in the testicular relative weight (Table 1).
|
Table 1: Variations of relative weight of testis and epididymis (X±SD) in male rabbits after 4 weeks of treatment. |
||||
|
|
Control |
CdSO4 |
Rs |
Cd-Rs |
|
Testis |
0.134± 0.006a* |
0.104± 0.017b* |
0.306± 0.006c* |
0.215± 0.010d* |
|
Epididymis |
0.021±0. 0017a* |
0.016± 0.009b* |
0.027± 0.009 |
0.02± 0.006 |
a: Control Vs CdSO4VsRsVs Cd-RS; b: Control Vs CdSO4; c: Control VsRs; d: Control Vs Cd-Rs
Testosterone level
The plasma testosterone concentration is significantly low in rabbits received cadmium compared with that of the control. In the combined group (Cd-Rs), testosterone level is also decreased, nevertheless, the effect is less important. However, the group treated with the radish seed extract shows a significant elevation compared to the other groups (Fig 1).
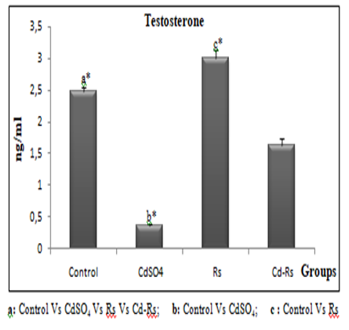
Figure 1:Variations of testosterone level (X±SD) in male rabbits after 4 weeks of treatment.
Sperm parameters
The results of the epididymal sperm analysis show a significant reduction in the speed, concentration and motility of spermatozoa of cadmium-treated group. The group (Rs) treated with the radish seed extract reveals a remarkable increase in all sperm parameters compared to the control. However, an improvement was recorded in combined treated group (Cd-Rs) compared to the control group. This improvement is moderate in speed and mobility, and significant in concentration of spermatozoa (Figs 2, 3 and 4).
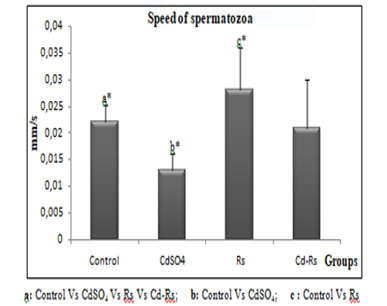
Figure 2: Variations of sperm speed (X±SD) in male rabbits after 4 weeks of treatment.
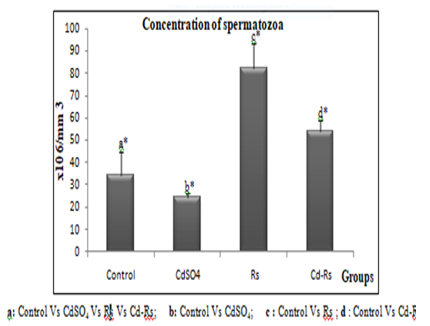
Figure 3:Variations of sperm concentration (X±SD) in male rabbits after 4 weeks of treatment.
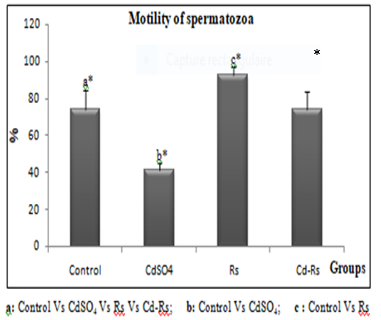
Figure 4: Variations of sperm motility (X±SD) in male rabbits after 4 weeks of treatment.
Histological study
Microscopic examination of the testis in the cadmium-treated rabbit shows certain architectural lesions of the testicular tissue. The magnification of x100, which gives a general view of the tissue structure (Fig 5.c), indicates an increase of lumen and intertubular spaces, as well as an absence of Leydig cells. The magnification of x400, gives a more precise and detailed view of the seminiferous tubules, which have a large and empty lumen spaces with a total absence of spermatozoa, and a decrease in the thickness of the tubular wall (Fig 5.d; Fig 5.b). Contrary, the control group has thicker walls and contains about seven layers of cells during differentiation and maturity.
On the other hand, the histological section of rabbit supplemented with radish seed extract has more regular spermatozoa (fig 5.e and f). Moreover, the lumens of the seminiferous tubules appear to be regular and full of mature spermatozoa, and the tubule walls are thicker and contain all the layers of cell differentiations. However, the histological section of rabbit testis received the combined treatment (fig 5.g and h) shows that the tubular structure in some parts is affected, but it is less accentuated compared to the cadmium group. Furthermore, the testicular structure of the combined treatment is manifested by a relative increase in diameter of some seminiferous tubules, narrowing walls, and an absence of mature spermatozoa in some others.
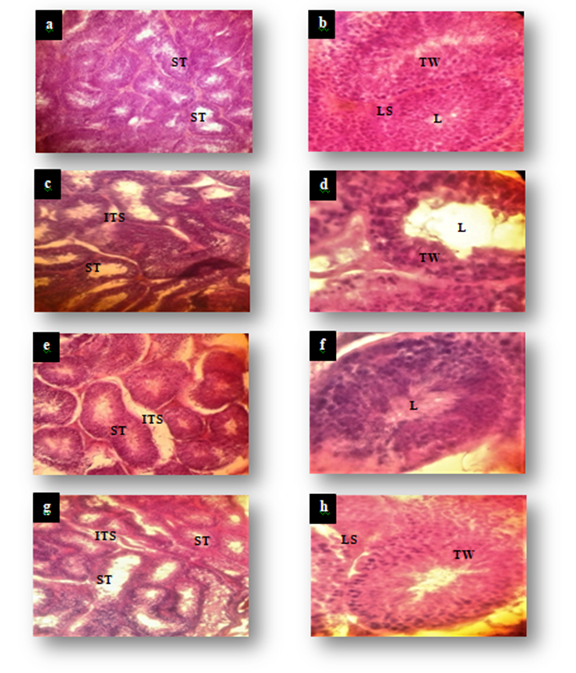
Figure 5: Histological sections of the testes; the control (a) and (b), (c) and (d) cadmium-treated group, (e) and (f) radish seeds extract treated group, (g) and (h) cadmium and radish seeds extract treated group. The (a), (c), (e) and (g) were magnified x100, while the (b), (d), (f) and (h) were magnified x400. LS: Leydig cells, L: Lumen, TW: Tubular wall, ITS: Intertubular spaces; ST: Seminiferous tubules.
Discussion
The relative weight of the reproductive organs has shown a remarkable reduction when rabbits have been exposed to chronic doses of cadmium, assuming that the metal is behind such effect. Cadmium was reported to damage blood vessels and increase lipids peroxidation (LPO), resulting in the destruction of cell membranes of the testicular germinal cells. [29] Interestingly, the radish seeds extract group showed a notable increase in the relative weight of testis and epididymis compared with the control, that is possibly explained by the fact that the seeds contain plant estrogen, which binds to the testicular receptors, therefore, contributing to feeding and increasing reproductive organs’ weight. [30] Moreover, an important increase in the reproductive organs was also recorded in the combined treatment group as compared to the cadmium-intoxicated rabbits that are perhaps related to the efficiency of radish seed components in neutralizing cadmium toxicity, like that of radish roots extract against mercury in Wistar rats. [31]
The decrease of plasma testosterone concentration by cadmium can be interpreted by the action of this metal on the hypothalamic-pituitary axis [32], leading to a disruption in androgens secretion, including testosterone synthesized by Leydig cells, which may subsequently affect the activity of other cells such as Sertoli cells [33] and the spermatozoa. It can be suggested that the decrease in testosterone level possibly has affected the process of spermatogenesis, which appeared through the obtained results. Radish seed extract when given alone, has boosted testosterone concentration and exceeded even that of the control group, in which [34] this increase has attributed to the ability of radish substances to stimulate the pituitary gland to secrete LH hormone, the latter stimulates testosterone production through Leydig cells. However, the result of testosterone concentration in the Cd-Rs group was close to that of the control, which could be explained by the presence of the necessary components, mainly the antioxidants like vitamin C, vitamin E, and flavonoids that have the capacity to combine with the free radicals generated by cadmium toxicity [31, 35].
The sperm speed, concentration and motility of rabbits have a clear decline in the cadmium-treated group, which is in-line with that of [36] who explained the decrease by the fact that cadmium cause oxidative stress by producing reactive oxygen species (ROS), which in turn stimulates testicular cell apoptosis. Even so [37] attributed this decrease to that the cadmium activates a large number of inflammatory mediators, thereby stimulating testis inflammation. Moreover, [38] related the decrease in the motility of spermatozoa to the ability of cadmium to replace calcium ions from their binding sites, whereas, [39] linked this decrease to the capability of cadmium to modifying the mitochondrial membrane, causing disturbances in the production of ATP required for sperm movement. On the other hand, the sperm parameters have recorded elevations in animals supplemented with radish seed extract more than those of the control do, which may be due to its components including vitamin C, vitamin E and flavonoids, a result agreed well with those obtained by [35]. Moreover, [40] revealed that radish can increase significantly the motility and sperm count in mice compared to the control. Furthermore, [34] explained this increase by the activation of estrogen hormones by radish seed components, leading to an improvement of sperm production. The results of sperm speed, concentration, and motility of the combined treatment are almost identical to the control, indicating the attenuating effect of radish seed extract towards cadmium intoxication. Previously, [31] interpreted similar results on rats by the fact that the antioxidant contents of radish root extract participate in reducing the oxidative stress through the reduction of ROS.
The testicular microscopic examination of cadmium treated rabbits at low magnification revealed irregular seminiferous tubules, increased intertubular spaces, expanded lumen tubules, and absence of Leydig cells in some locations, which resulted from necrosis and spermatozoa death as cadmium was reported to damage cell membranes, leading to sperm decease. [33] However, at high magnification, the histological sections showed a retraction of the tubular walls, and an absence of one or two stages of spermatogenesis, suggesting that cadmium has been accumulated inside the cells. [41] Studies showed that Cd causes dose-dependent apoptotic and/or necrotic cell deaths in spermatogenic epithelium. [42] Such effect could be explained by the increase of lipid peroxidation through the generation of noxious radicals such as superoxide anion radicals, hydroxyl radicals, nitric oxide and hydrogen peroxide. [43] However, ROS have been highlighted, as a mediator factor of apoptotic cell death in Cd-induced oxidative stress. [43]
The histological sections of rabbit testis supplemented with radish seeds are characterized by tubules with thick walls and full of spermatozoa, in which their number exceed that of the control. This result is in agreement with the study of [44] who found clear presence of all stages of spermatogenesis, and the germ cell epithelia within the seminiferous tubules were notably increased in radish seeds-treated group. Moreover, radish seed increased libido as well as steroidogenesis and spermatogenesis. The findings confirm that radish seeds contain vitamins and minerals that contribute to the regulation of spermatogenesis, and lead to an increase in seminiferous wall thickness and an elevation in mature spermatozoa. [45] Similarly, [31] showed that the supplementation of Raphanussativus juice ameliorates the histological architecture of reproductive organs like the testis and epididymis and increases the number of sperms. Even though, the combined administration indicated the absence of spermatozoa in some seminiferous tubules, with a decrease in walls’ thickness that certainly owed to cadmium intoxication and sperm death.[41]However, it seems that radish seeds can protect the reproductive organ partially since this plant contains various antioxidants such as anthocyanins[46], and protect the testis against oxidative stress produced by cadmium through the scavenging of free radicals. [47] Radish is important source of vitamin C and sulfur[48], which possibly scavenge the free radicals and thus protects sperms from oxidative damage. [49, 50] Moreover, ascorbic acid was reported to be essential for testicular differentiation, integrity and steroidogenic functions. [51, 52]
Conclusion
This study indicates the toxic effect of cadmium, which has disturbed sperm parameters (speed, concentration and motility), the level of testosterone, as well as the degeneration in the testicular architecture of rabbits after one-month exposure. Radish seeds when administrated alone have revealed beneficial activities to all reproductive markers. However, the combined supplementation of Cd-Rs showed a remarkable attenuating role of radish seeds towards cadmium induced toxicity.
References
- Yari A, Sarveazad A, Asadi E, RaoufSarshoori J, Babahajian A, Amini N, et al. Efficacy of Crocus sativus L. on reduction of cadmium-induced toxicity on spermatogenesis in adult rats. Andrologia. 2016; 48(10): 1244–1252.
- Cannino G, Ferruggia E, Luparello C, Rinaldi AM. Cadmium and mitochondria. Mitochondrion. 2009; 9: 377–384.
- Manca D, Ricard AC, Trottier B, Chevalier G. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology. 1991; 67: 303–32
- Satarug S, Nishijo M, Lasker JM, Edwards RJ, Moore MR. Kidney dysfunction and hypertension: role for cadmium, P450 and hemeoxygenases? Tohoku. J. Exp. Med. 2006; 208: 179–202.
- Liu J, Cheng ML, Yang Q, Shan KR, Shen J, Zhou Y, et al. Blood metallothionein transcript as a biomarker for metal sensitivity: low blood metallothionein transcripts in arsenicosis patients from Guizhou. China. Environ. Health. Perspect. 2007; 115: 1101–1106.
- Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature. 1956; 177: 103
- Young RJ, Bodt BA, Heitkamp DH. Action of metallic ions on the precocious development of rabbit sperm motion patterns that are characteristic of hyperactivated motility. Mol. Reprod. Dev. 1995; 41: 239-248.
- Gunnarsson D, Nordberg G, Lundgren P, Selstam G. Cadmium-induced decrement of the LH receptor expression and cAMP levels in the testis of rats. Toxicology. 2003; 183: 57-63.
- Zhao LL, Ru YF, Liu M, Tang JN, Zheng JF, Wu B, et al. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS One. 2017; 12(11).
- Boscolo P, Sacchettoni-Logroscino G, Ranellete FO, Gioia A, Carmigani M. Effects of long term cadmium exposure on the testes of rabbits. Ultrastructural study. Toxicol. Lett. 1985; 24: 145-149.
- Kime DE, Ebrahimi M, Nysten K, Roelants I, Moore HDM, Ollevier F. Use of computer assisted sperm analysis (casa) for monitoring the effects of pollution on sperm quality of fish; application to effects of heavy metals. Aquatic. Toxicology. 1996; 36(1): 223-237.
- Rurangwa E, Roelants I, Huyskens G, Ebrahimi M, Kime DE, Ollevier F. The minimum acceptable spermatozoa to egg ratio for artificial insemination and the effects of heavy metal pollutants on sperm motility and fertilization ability. In the african catfish (clariasgariepinus. Journal of Fish Biology. 1998; 53: 402-413.
- Waalkes MP. Cadmium carcinogenesis. Mutat. Res. 2003; 533: 107–120.
- Prozialeck WC, Edwards JR. Mechanisms of cadmium induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012; 343: 2–12.
- Romina M, Lasagni, Vitar RM, Reides CG, Ferreira SM, Llesuy SF. The protective effect of Aloysiatriphylla aqueous extracts against brain lipid-peroxidation. Food and Function. 2014; 5(3): 557-563.
- Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY. Protective roles of onion and garlic extracts on cadmiuminduced changes in sperm characteristics and testicular oxidative damage in rats. Food. Chem. Toxicol. 2008; 46: 3604-3611.
- Obioha UE, Suru SM, Ola-Mudathir KF, Faremi TY. Hepatoprotective potentials of onion and garlic extracts on cadmium-induced oxidative damage in rats. Biol. Trace. Elem. Res. 2009; 129: 143–156.
- OlalekanLawal A, Lawal AF, Ologundudu A, Adeniran OY, Omonkhua A, Obi F. Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. J. Toxicol. Sci. 2011; 36: 549–557.
- Ola-Mudathir FK, Suru SM. Onion and garlic extracts as potential antidotes for cadmium-induced biochemical alterations in prostate glands of rats. Andrologia. 2015; 47: 1075–1082.
- Oyinloye BE, Adenowo AF, Osunsanmi FO, Ogunyinka BI, Nwozo SO, Kappo AP. Aqueous extract of Monodoramyristica ameliorates cadmium-induced hepatotoxicity in male rats. Springer Plus. 2016; 5: 641.
- Onoshe S, Madusolumuo MA. Effect of hexane seed extract of Nigella sativa on cadmium induced renal dysfunction in rats. Am. J. Res. Commun. 2014; 2: 158-171.
- Elgaml SA, Hashish EA. Clinicopathological studies of Thymus vulgaris extract against cadmium induced hepatotoxicity in albino rats. Glob. J. Pharmacol. 2014; 8: 501-509.
- Tarasub N, Junseecha T, Tarasub C, Ayutthaya WD. Protective efects of curcumin, vitamin C, or their combination on cadmium-induced hepatotoxicity. J. Basic. Clin. Pharm. 2012; 3: 273–281.
- Eybl V, Kotyzova D, Koutensky J. Comparative study of natural antioxidants-curcumin, resveratrol and melatonin-in cadmium-induced oxidative damage in mice. Toxicology. 2006; 225: 150–156.
- Banihani SA. Radish (Raphanussativus) and Diabetes. Nutrients. 2017 ; 9(9) : 1014.
- He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu. Rev. Food. Sci. Technol. 2010 ; 1 : 163-187.
- Zerrouki N. Caractérisationd’une population locale de lapin en Algérie: évaluation des performances de reproduction des lapines en élevagerationnel. ThèseDoctorat. Université de TiziOuzou (Algérie). 2006; 131.
- World Health Organisation (WHO). Analyse human sperm and interaction with sperm cervical mucus. Ed. INSERM. 1993; 55-56.
- Anietie A, Odamesan A, James A. Semen traits, testicular morphometry and histopathology of cadmium-exposed rabbit bucks administered methanolic extract of Phoenix dactylifera fruit. J. Maringá. 2017; 39(2): 207-215.
- Hess R, Bunick D, Lee K, Bahr J, Taylor J, Korach K, Lubahn D. A role for oestrogens in the male reproductive system. Nature. 1997; 390: 509-512.
- Siouda W. The detoxification of mercury by Algerian medicinal plants (Urticadioica and Raphanussativus) in Wistar rats. Thesis doctorat, University of BadjiMokhtar- Annaba. 2016.
- Kime D. The effects of pollution on reproduction in fish. Reviews in Fish Biology and Fisheries 1995; 5(1): 52-96.
- Ebrahimi M, Taherianfard M. The effects of heavy metals exposure on reproductive systems of cyprinid fish from Kor River. Journal of Fisheries Sciences. 2011; 10 (1): 13-24.
- Mehraban F, Jafari M, AkbartabarToori M, Sadeghi H, Joodi B, Sadeghi H. Effects of date palm pollen (Phoenix Mostafazade, dactylifera L.) and Astragalusovinus on sperm parameters and sex hormones in adult male rats. Iran. J. Reprod. Med. 2014; 12: 705-712.
- El-Tohamy M, El-kady R. The beneficial effects of Nigella sativa, Raphanussativus and Erucasativa seed cakes to improve male rabbit fertility, immunity and production. J. Am. Sci. 2010; 6: 1247-1255.
- Kim J, Soh J. Cadmium-induced apoptosis is mediated by the translocation of AIF to the nucleus in rat testes. Toxicol. Lett. 2009; 188(1): 45-51.
- De Freitas M, Dalmolin L, Oliveira L, Da Rosa Moreira L, Roman SS, Soares F. Effects of butane-2,3-dione thiosemicarbazoneoxime on testicular damage induced by cadmium in mice. J. Toxicol. Sci. 2012; 37(5): 899-910.
- Okuno M, Morisawa M. Effects of calcium on motility of rainbow trout sperm flagella demembranated with Triton X-100. Cell. Motil. Cytoskel. 1989; 14(2): 194-200.
- Xu L, Wang S, Yang X, Wang S. Effects of cadmium on rat sperm motility evaluated by computer assisted sperm analysis. Biomed. Environ. Sci. 2001; 14(4): 312-317.
- Hassan AM, Alam SS, Abdel-Aziem SA, Ahmed KA. Benzo-a-pyrene induced genotoxicity and cytotoxicity in germ cells of mice: Intervention of radish and cress. Journal of Genetic Engineering and Biotechnology. 2011; 9(1): 65-72.
- Alfiah H, Hanna P, Inayatul K, Dwi W, Sugiharto. Histopathological assessment of cadmium effect on testicles and kidney of Oreochromisniloticus in different salinity. AIP Publishing. 2016; 10: 1063.
- Gupta Sen R, Kim J, Gomes C, Oh S, Park J, Im WB, et al. Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol. Cell. Endocrinol. 2004; 221: 57.
- Stohs SJ, Bagchi D, Hassoun E, Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2001; 20: 77.
- Elgenaidi AR. Effects Of Libyan Traditional Plants On The Reproductive System Of Male And Female Rats. Thesis of the requirements for the degree of Philosophiae Doctor (PhD). Department of Medical Biosciences, Faculty of Natural Sciences University of the Western Cape. 2015.
- Breininger E, Beorlegui N, O’Flaherty C, Beconi M. Alpha-tocopherol improves biochemical and dynamic parameters in cryopreserved boar semen. Theriogenology. 2005; 63: 2126-2135.
- Kong JM. Analysis and biological activities of anthocyanins, Phytochemistry. 2003; 64: 923-933.
- Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Letters. 2008; 269: 281-290.
- Naghibi F, Pourmorad F, Honary S, Shamsi M. Decontamination of water polluted with phenol using Raphanussativus root. Iranian Journal of Pharmaceutical Reseach. 2003; 2: 29-32.
- Castllini C, Lattaioli P, Dal BA, Minelli A, Mugnai C. Oxidative status and semen characteristics of rabbit buck as affected by dietary vitamin E, C and n-3 fatty acids. Reprod. Nutr. Dev. 2003; 43: 91-103.
- Yousef MI. Protective effect of ascorbic acid to enhance reproductive performance of male rabbits treated with stannous chloride. Toxicology. 2005; 207: 81-9.
- Dawson EB, Harris WA, Powell LC. Relationship between ascorbic acid and male fertility. World. Rev. Nutr. Diet. 1990; 62: 1-26.
- Luck MR, Jeyaseelan I, Scholes RA. Ascorbic acid and fertility. Biol. Reprod. 1995; 52: 262-266.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
