Effect of chronic Allopurinol therapy on Thyroid function in patients with urate stones
Ibrahim M. Faisal 1, Marwan M. Merkhan 2*, Hani M. Almukhtar 2
1 Department of Pharmacology, College of Medicine, University of Mosul, Mosul, Iraq. 2 Department of Pharmacology and toxicology, College of Pharmacy, University of Mosul, Mosul, Iraq.
ABSTRACT
A xanthine-oxidoreductase enzyme is responsible for purine metabolism in our body; converting purine to its end product in the form of uric acid. Inhibition of this enzyme will then reduce uric acid in gout patients, however, it could have some side effects on other tissues and organs. The present study aims to identify the effect of long-term use of allopurinol in modulating the thyroid function in a patient with urate-type renal stone. The result revealed that allopurinol reduced the levels of free form of thyroid hormone and elevate TSH level with no effect reported with the bound thyroid hormones; these results were confirmed by ultrasonography results obtained for the patient before and after allopurinol therapy showing enlarged thyroid gland, change in echogenicity, and increased blood supply with nodule formation in few of them. These study has concluded that allopurinol could induce a state of subclinical hypothyroidism.
Keywords: gout, xanthine oxidase, uric acid, thyroid hormones.
Introduction
Structurally, allopurinol is a purine analog compound and is described as a first-line uricosuric agent for the treatment of hyperuricemia and gout reducing the recurrence of gout attacks and decreasing the amount of monovalent sodium urate concentration which could lead to tissue injury, crystals and inflammation [1-4]. Gout is a common disease and is associated with a high rate of morbidity, resulting in a greater increase in allopurinol use [5, 6]. A new anti-gout medication has been recently introduced in clinical practice (benzbromarone, lesinurad, and probenecid), however, these medications are less popular in comparison to allopurinol [7]. The
hypouricaemic effects of allopurinol have been limited by renal impairment which is common in gout patients, however, other uricosuric agents have more deleterious side effects particularly hepatic toxicity associated with benzbromarone; which led to its withdrawal from the markets [8]. Elevated thyroid-stimulating hormone (TSH) level in a febuxostat-treated patient has been reported after long term therapy [9, 10]. Therefore, the febuxostat medication leaflets carry a caution of avoidance in thyroid disease patients, however, there is only limited data regarding the negative impact of allopurinol in thyroid patients. Therefore this study was designed to determine the effect of long-term allopurinol use on the thyroid gland in comparison to baseline (before allopurinol therapy) and healthy patients using ultrasonography and analytical test for diagnosis. Unfortunately, limitation of the number of patients with active urate stone and poor patient adherence to therapy has hampered the progress of the study leading to the enrollment of only limited numbers of patients in the study.
Methods
A total of 15 renal stone (9 male and 6 female) patients were enrolled in this study, they were treated with allopurinol therapy for 6 months. Another group involved twenty-two healthy volunteers (14 male and 8 female), were also enrolled in the study as a control group for comparison. The demographic parameters were mentioned in Table 1 below. A consent form of agreement has been taken from all subjects.
The study design was a case-control study and the patients were excluded from it if they had a history of any chronic disease (including, thyroid disease, hypertension, angina pectoris, myocardial infarction, heart failure, renal or hepatic insufficiency or those taking any chronic medication; particularly amiodarone. Pregnant women and lactating mothers were also excluded from the study.
For laboratory analysis of biochemical parameters, Five milliliters of venous blood was obtained from patient and control subjects after 12-hours fasting, The blood was left at room temperature to clot and after centrifugation, the serum was separated in plain tube, samples were collected and stored in freezers (-20°C) to be ready for analysis. Frozen samples were thawed and analyzed. Thyroid hormones; total triiodothyronine (total T3), thyroxine (total T4), free triiodothyronine (fT3), free thyroxine (fT4) and TSH were measured using Enzyme-Linked Fluorescent Assay technique (minivans, France), by using thyroid hormone kit (Biomerieux company, France). Further confirmation of the study was achieved by sending the patient into the ultrasound specialist for checking thyroid gland structure (Philips HD-11 Digital Ultrasound Machine).
|
Table 1. Demographic characteristics of the studied groups. |
||
|
Parameters |
Control group (No.=22) |
Allopurinol group(No.=15) |
|
Age (years) |
40.91±2.2 |
39.41±3.8NS |
|
BMI (kg/m2) |
27.9±3.5 |
28.2±3NS |
|
Duration of treatment |
…….. |
6 months |
|
Gender (male/Female) |
14/8 |
9/6 |
NSNon significant differences as compared to the same parameter in the control group (P > 0.05).
Data were represented as the mean ± standard deviation. Comparisons between groups were conducted using the t-test. P<0.001 was considered to describe a statistically significant difference. Statistical results were obtained using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results and Discussion
Its recommended by the European Medicine Agency to avoid using allopurinol in patients with thyroid disease due to occasional investigation of elevated TSH in febuxostat and allopurinol treated patients [2, 11], however, no further studies is determining the long term use of allopurinol on the peripheral thyroid parameter including the levels of free form. Hereby, we aimed to identify the effect of long-term use of allopurinol on the peripheral thyroid hormone axis as a function of measuring the level of free and bound thyroid hormones together with TSH; the importance of this study is due to the coincidence of thyroid abnormalities and impaired uric acid metabolism together with the fatal cardiovascular disease.
In presence of small scale evidence of the impact of inhibition of xanthine oxidoreductase on thyroid function by febuxostat; in the line with that allopurinol could carry such deleterious or protective effects on thyroid gland [7]. A retrospective and prospective study reported elevated TSH levels in hyperuricaemic patients on uricosuric agents [8]. Moreover, a concomitant association between hyperuricaemic and hypothyroidism has been demonstrated; allopurinol could be an overt causative agent in the enrolled subjects in the study [9, 12]. In the present study, we found out that allopurinol has induced a significant (p<0.001) reduction in the level of the free forms of thyroid hormones [(fT3=1.5±0.6), (fT4=8.3±1) pmol/l] with no changes in the level of the bound form [(T3=1.5±0.7), (T4=8.3±1.1) nmol/l] confirmed by elevated TSH levels [(TSH=6.2±0.6)mIU/l] (Figure 1); the present finding were confirmed by ultrasonography parameters which shows an impairment of thyroid tissues structure, shape, size, and echogenicity (Figure 2). Nevertheless, this finding is controversial due to the association between overt hypothyroidism and hyperuricemia in allopurinol negative patients [13, 14]. The present study shows that increased blood flows to both thyroid gland lobes in hyperuricaemic patients even before the association of allopurinol therapy (Table 2). The demographic character between the control and patients was acceptably matched; to avoid any mismatching between age and BMI (Table 1). The duration of treatment of allopurinol was 6 months and the dose was 300mg/day.
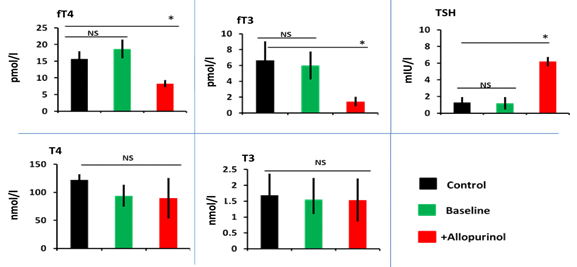
Figure 1. Allopurinol induced subclinical hypothyroidism through reducing free T3 and free T4 and elevation of TSH in comparison to control healthy or same subject baseline level, data expressed as mean±SD, *p<0.001, NS=non-significant.
In the present study; the elevated TSH levels were associated with a reduction in free form of thyroid levels and there were no obvious changes in the bound form, these sign and symptoms are attributable to the subclinical hypothyroidism; these results were in agreement with a study done on dog treated by allopurinol resulting in increased TSH levels with no change inbound form of T4 and T3 hormone levels [13, 14], confirmed by data obtained from ultrasonography parameters (Figure 2 and Table 2).
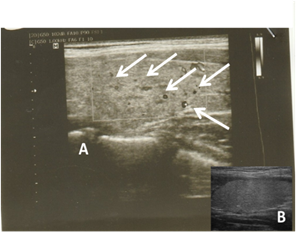
Figure 2. a representative ultrasonogram of patient before (B) allopurinol and after (A) allopurinol indicating the presence of nodules (arrows) in allopurinol-treated in comparison to baseline (See also table 2).
Nevertheless, a study conducted on the role of therapeutic intervention on thyroid hormones showed a negative correlation between fT4 and TSH [15-18]. To sum up; the present study observed an increase in TSH, fT3, and fT4 levels, with no changes associated with T4 and T3 levels or clinical manifestations apart from ultrasonography parameter changes. Its recommended to identify the correlation between TSH (and subsequently downstream thyroid axis) therefore further studies are needed.
|
Table 2. Ultrasonography parameters for subjects before and after allopurinol therapy. |
||
|
Ultrasound Parameters |
Allopurinol |
|
|
|
Before |
After (Male/female) |
|
Size |
Normal |
Increase (1/3) |
|
Echogenicity |
Normal |
Increase (15) |
|
Nodule |
None |
Presence (2/2) |
|
Blood flow |
Increased |
Increase (15) |
|
Anechoic area |
None |
Scattered (2/3) |
Conclusions
Long-term use of allopurinol induced an elevated level of TSH, however, there is no alteration in the levels of the bound thyroid hormones with a reduction in free form. These results could suggest that long-term use of Allopurinol may lead to subclinical hypothyroidism and such patients may require annual screening of their thyroid function particularly in endemic countries with goiter and their bound thyroid hormone levels do not predict the risk.
Acknowledgments
The authors are very grateful to the University of Mosul/College of Medicine and College of Pharmacy for their provided facilities, which helped to improve the quality of this work. They also thank the scientific committee in the department of pharmacology and toxicology of the college of the pharmacy/University of Mosul.
References
- Khayoon, W. S., Al-Abaichy, M. Q., Jasim, M., Al-Hamadany, M. A. Spectrophotometric determination of Allopurinol in tablet formulation. Journal of Physical Science, 2008; 19(2), 23-30.
- European Medicines Agency. EPACHMP assessment report for Adenuric, 2008. http://www.ema.europa.eu/docs/en.
- Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis care & research. 2012 Oct;64(10):1431-46.
- Sudjarwo FE, Prawita A, Wulandari WP. The Effectiveness of Indonesian Tradional Therapy from Blimbing Wuluh Leaf Extract in Reducing Uric Acid in Mus Musculus. International Journal of Pharmaceutical Research & Allied Sciences. 2018 Jul 1;7(3).
- Dalbeth, N., Merriman, T.R., Stamp, L.K. Gout. Lancet, 2016. doi: 10.1016/S0140-6736(16)00346-9.
- Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nature reviews rheumatology. 2015 Nov;11(11):649.
- Makay O, Yenisey C, Icoz G, Simsek NG, Ozgen G, Akyildiz ME, Yetkin E. The role of allopurinol on oxidative stress in experimental hyperthyroidism. Journal of endocrinological investigation. 2009 Sep 1;32(8):641-6.
- Erickson AR, Enzenauer RJ, Nordstrom DM, Merenich JA. The prevalence of hypothyroidism in gout. The American journal of medicine. 1994 Sep 1;97(3):231-4.
- Perez-Ruiz F, Dalbeth N, Schlesinger N. Febuxostat, a novel drug for the treatment of hyperuricemia of gout. Future. 2008 Oct;3(5):421-7.
- Najari F, Dadpour B, Vahabzadeh M, Alizadeh-Ghamsari A, Mousavi SR, Kial IB. Value of Blood Lactate and Tsh in Predicting Length of Hospitalization and Outcome of Poisoned Patients Admitted to the Icu. Pharmacophore. 2018 May 1;9(3):7-12.
- Alnahdi HS. The Possible Ameliorative Mechanisms of Curcumin and/or Coenzyme Q10 Against Hyperthyroidism Induced Liver Damage in Rats. Entomology and Applied Science Letters. 2018 Mar 3;5(1):7-16.
- See LC, Kuo CF, Yu KH, Luo SF, Chou IJ, Ko YS, Chiou MJ, Liu JR. Hyperthyroid and hypothyroid status was strongly associated with gout and weakly associated with hyperuricaemia. PloS one. 2014 Dec 8;9(12):e114579.
- Perez‐Ruiz F, Herrero‐Beites AM, Carmona L. A two‐stage approach to the treatment of hyperuricemia in gout: The “dirty dish” hypothesis. Arthritis & Rheumatism. 2011 Dec;63(12):4002-6.
- Giordano N, Santacroce C, Mattii G, Geraci S, Amendola A, Gennari C. Hyperuricemia and gout in thyroid endocrine disorders. Clinical and experimental rheumatology. 2001 Nov 1;19(6):661-6.
- M Merkhan M. The effects of glibenclamide on thyroid function tests in type 2 diabetic patients. Iraqi Journal of Pharmacy. 2013 Dec 28;13(2):55-61.
- Saridomichelakis, M. N., Xenoulis, P. G., Chatzis, M. K., Kasabalis, D., Steiner, J. M., Suchodolski, J. S., Petanides, T. Thyroid function in 36 dogs with leishmaniosis due to Leishmania infantum before and during treatment with allopurinol with or without meglumine antimonate. Veterinary parasitology, 2013; 197(1-2), 22-28.
- Stamp, L. K., Taylor, W. J., Jones, P. B., Dockerty, J. L., Drake, J., Frampton, C., Dalbeth, N. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis & Rheumatism, 2012; 64(8), 2529-2536.
- Komoriya, K., Hoshide, S., Takeda, K., Kobayashi, H., Kubo, J., Tsuchimoto, M., ... Kamatani, N. Pharmacokinetics and pharmacodynamics of febuxostat (TMX‐67), a non‐purine selective inhibitor of xanthine oxidase/xanthine dehydrogenase (NPSIXO) in patients with gout and/or hyperuricemia. Nucleosides, Nucleotides and Nucleic Acids, 2004; 23(8-9), 1119-1122.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
