Cost optimization in the drug treatment of the inpatients with Schizophrenia
Elena Maksimkina1, Larisa Vaskova1*, Ivan Krysanov2, 3, Marina Tiapkina1, Anastasiya Mancheva4
1 Sechenov First Moscow State Medical University, 8-2 Trubetskaya str., Moscow, 119991, Russian Federation. 2 Medical Institute of Continuing Education, Moscow State University of Food Production, 11 Volokolamskoe highway, Moscow, 125080, Russian Federation. 3 National Research Institute of public health named after N. A. Semashko, 12 Vorontsovo poles str., building 1, Moscow, 105064, Russian Federation. 4 "KorolevPharm" (LLC), 4 Pionerskaya Str., Korolev, Moscow Region, 141074, Russian Federation.
ABSTRACT
Purpose: The work has been aimed at developing an interactive pharmacoeconomic tool that allows determining the cost and consumption of the drugs used for treating schizophrenia and optimizing the cost based on the results of the comprehensive pharmacoeconomic analysis. Materials and methods: The pharmacoeconomic methods used were as follows: the Cost of Illness analysis, the Impact on the Budget analysis, the Cost and Efficacy analysis, the Cost Minimization analysis, and the ATC/DDD methodology of the WHO. Results: The developed software is a tool for pharmacoeconomics managers. The user enters the initial data for each patient, such as gender, age, treatment regimen, and duration of hospitalization. For calculating the cost, the data about the purchase price of the drugs are entered. The application allows calculating the amount of the drugs taken by a patient, consumption (NDDD/100 bed-days), and the cost of drug treatment. The cost can be summarized by certain criteria: by international nonproprietary or commercial names, by the groups of drugs, by the hospitalization unit, by the disease code (international classification of diseases (ICD)-10), which allows controlling the costs of medical organizations and their structure. The obtained data can be used for the Cost of Illness analysis, the Impact on the Budget analysis, the Cost and Efficacy analysis, the Cost and Practicability analysis, and the Cost Minimization analysis. For this purpose, the data about the efficacy and the quality of life are provided by the user. Conclusion: The application allows healthcare professionals to estimate, using the pharmacoeconomic approach, the cost of inpatient schizophrenia drug treatment for both a single patient and the studied cohort of patients, and to manage the psychiatric medical organizations' costs amount and structure.
Keywords: Cost and Efficacy analysis, Cost Minimization analysis, Impact on the Budget analysis, software, pharmacoeconomic analysis
Introduction
Schizophrenia is one of the most common and costly mental disorders in the world. For instance, its incidence rate in the world ranges from 0.5 to 2 %; in the Russian Federation in 2018, 539,644 patients applied for neuropsychiatric care, thus, the incidence rate was about 0.4 % [1]. The emergence of new expensive drugs increases the cost of drug treatment, which is a problem for public health care. As such, according to domestic studies, the economic costs may reach 0.5 % of the GDP or up to 40 % of the budget allocated for mental health care [2].
Healthcare reforms and changes in technology, government policy, and consumer expectations are revolutionizing relationships with key stakeholders, impacting operations in unforeseen ways [3]. Pharmacy and Pharmacists play an essential role in well-being and health care [4]. Providing pharmaceutical care for the population is regulated by Federal Law No. 323 "On the Basics of Health Care for the Citizens in the Russian Federation" dated 21.11.2011 [5]. According to the Federal Law, the state health care policy is aimed at satisfying the needs of the population and institutional consumers not only ineffective and safe drugs but also in the most cost-efficient ones that ensure the highest possible quality of life. Thus, the main goal of the health care economy is the reasonable choice of rational utilization of financial resources [6].
The need for the development of pharmacoeconomics is determined by the growing costs in health care, by the emergence of new drugs that have significantly changed the course of the diseases, and by the emergence of the fundamentally new medical technologies that increase not only the life expectancy but also its quality [7].
With the emphasis on patient-oriented care, the practice of pharmacy has undergone a marked
Evolution [8]. According to the regulations in the field of pharmacoeconomics and the outcomes of the studies, full economic evaluation includes the Cost Minimization, the Cost and Efficacy, and the Cost and Practicability analyses [9, 10]; in the authors’ manuals [11], the main methods are the following: the Impact on the Budget analysis, the Cost and Efficacy analysis, and the Cost Minimization analysis. Pharmacoeconomic modeling, which expands the practical use of pharmacoeconomic assessment, is indispensable. The above methods of pharmacoeconomics are the most popular today in public health practice [12].
Currently, there is a need for monitoring and optimizing the cost of drug treatment for patients with schizophrenia. Software development using methods of the pharmacoeconomic analysis will allow the rational use of limited economic resources of psychiatric hospitals.
Today there are several applications used in pharmacoeconomic studies, such as PharmCompile, PharmSuite, and RAOM (Russian Association of Oncological Mammology) online calculator.
The PharmCompile application is intended for automated ABC and VEN analyses of a range of drugs in medical organizations and allows the retrospective analysis of the drug lists from the perspective of the cost, vital importance, and frequency of drug consumption [12].
The PharmSuite application allows performing ABC and VEN analyses of the drugs separately by international names and trade names concerning the dosage form and registration of side effects [13].
The RAOM online calculator is intended for the pharmacoeconomic analysis of the electronic RAOM recommendations and allows calculating the cost of treatment and assessing the need for drugs (in packages) based on the entered characteristics and patient models. It also allows saving user-profiles and performing the Impact on the Budget pharmacoeconomic analysis [14].
However, the above applications include individual elements of pharmacoeconomic analysis and are intended for assessing the cost of treatment for patients with other nosologies. Thus, the development of software for integrated pharmacoeconomic analysis in psychiatric practice is relevant.
The objectives of the study were the following:
- Developing an algorithm for automating the process of calculating the drug consumption and the cost of psychiatric inpatient treatment of the patients with schizophrenia;
- Developing an approach to cost optimization based on the methods of Impact on the Budget, Cost, and Efficacy, and Cost Minimization pharmacoeconomic analyses for inpatient care.
Methods
A toolkit has been developed for cost modeling with the use of the Cost Microcalculation method for pharmacotherapy, the Cost and Efficacy, and the Cost Minimization analyses, and assessing the impact on the Budget using a continuous sample of the patients with schizophrenia hospitalized to the psychiatric hospital in Moscow (the International Classifier of Diseases code is F20.0). On the example of the studied sample (n = 198), the following parameters were studied: the demographic information (gender, age), the information about the drugs prescribed (dosage, dosage frequency, and duration of therapy), and the duration of patients' hospitalization.
The drug consumption was calculated and analyzed based on the ATC/DDD methodology of the World Health Organization (WHO). At the institutional level, the information about drug consumption in the hospital segment was expressed as DDD/100 bed-days. The sources of the information about the number of prescribed drugs were the patient’s medical history; the source of defined daily doses (DDD) was the website of the WHO for drug statistics methodology (https://www.hocc.no/atc_ddd_index/). The cost of the drugs was assessed from the state procurement data provided by the hospital.
Results
To perform a pharmacoeconomic study and develop technical specifications for creating the software, 198 case histories of the patients with schizophrenia (ICD code F20.X) hospitalized to the Korsakov Psychiatry Clinic were analyzed.
The developed pharmacoeconomic calculator has three blocks: the block for entering the initial information, the data array, and the results interpreting block. To assess the cost of drug treatment, two approaches were implemented in the calculator: the pharmacoeconomic analysis for the selected cohort of patients concerning the individual characteristics of the patients and the drug treatment regimens (Figure 1) and the independent pharmacoeconomic analysis according to the standardized treatment regimens (Figure 2).
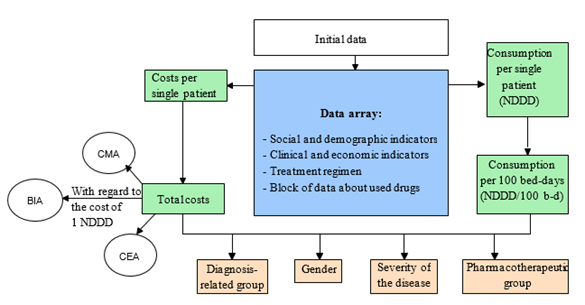
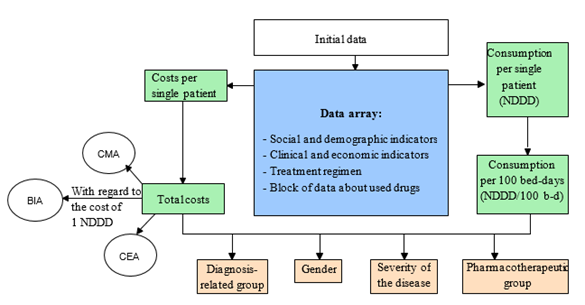
Figure 1: The pharmacoeconomic analysis based on the individual treatment regimens and the characteristics of the patient
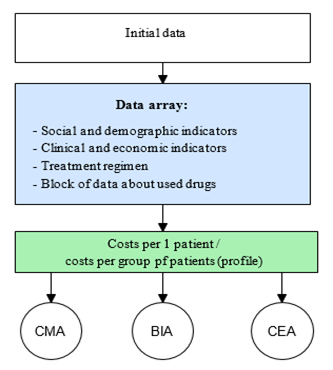
Figure 2: The independent pharmacoeconomic analysis (for standardized treatment regimens)
Functionally, the pharmacoeconomic calculator allows two independent studies. For the pharmacoeconomic analysis concerning the individual treatment regimens for the patients with schizophrenia, the following information is entered:
- information about the patient,
- information about the treatment regimen.
The entered information is used for generating data arrays: the database for each patient and the database of the drugs used.
Based on the generated data arrays, the cost and consumption of drugs are calculated, and their pharmacoeconomic assessment is made.
The first block involves entering information about the patient: case history number, gender, age, disability group, unit, disease code per international classifier (ICD-10), and duration of hospitalization.
Also, the information about the drug treatment is entered: the international nonproprietary number (INN) and the trade name are selected, and the daily dose and the days of administration are entered.
The second block is represented by data arrays: the database for the prescribed drugs, into which the user enters the group of drugs, the INN, the trade name, the dosage, the dates of drug administration, and the database that contains information about the nomenclature of the drugs used: the name, the group, the dosage, the drug form, the cost of packing, and the WHO DDD.
The data entered for each patient are grouped into profiles and contain the following information: the number of drug prescriptions and the cost of drug treatment.
The third block contains clinical and economic indicators: the total, the average (arithmetic mean), and the minimum and maximum duration of hospitalization in days, the number of drug prescriptions, and the cost in rubles (Table 1, 2).
|
Table 1: The Cohort Characteristic window: 3rd block |
||||
|
Clinical and economic indicators |
Total |
Min |
Max |
Average |
|
Duration of hospitalization, days |
13,986 |
3 |
217 |
70.64 |
|
The number of prescriptions |
1,089 |
1 |
19 |
5.5 |
|
Cost, rubles |
1,400,324.19 |
50.05 |
85,095.32 |
7,072.34 |
Table 2: The Cohort Characteristic window: 1st block
|
Sociodemographic characteristics |
General cohort |
male |
female |
|
The number of patients |
198 |
136 |
62 |
|
The average age |
39.5 |
35.9 |
47.4 |
|
The number of patients with disabilities |
118 |
81 |
37 |
|
including group 1 |
29 |
18 |
11 |
|
including group 2 |
81 |
57 |
24 |
|
including group 3 |
8 |
6 |
2 |
|
Without disability |
80 |
55 |
25 |
The data about the socio-demographic characteristics of the patients (the total number of patients in the studied cohort, their average age, the number of the patients with disabilities (including group 1, 2, and 3)) are presented both totally for the studied cohort, and in the subgroups identified by the gender.
This window also contains information about the patients' allocation to clinical and statistical groups by the main diagnosis according to ICD-10, based on the type of the disease (Table 3).
|
Table 3: The Cohort Characteristic window: 1st block - distribution of patients |
|||
|
Clinical statistics group |
ICD-10 code |
The absolute number of cases |
Share, % |
|
Paranoid schizophrenia |
F20.0 |
156 |
78.88 |
|
Hebephrenic schizophrenia |
F20.1 |
5 |
2.53 |
|
Catatonic schizophrenia |
F20.2 |
3 |
1.52 |
|
Undifferentiated schizophrenia |
F20.3 |
5 |
2.53 |
|
Post-schizophrenic depression |
F20.4 |
2 |
1.01 |
|
Simple schizophrenia |
F20.6 |
8 |
4.04 |
|
Other schizophrenia |
F20.8 |
19 |
9.60 |
For each group, information is provided about the patients' distribution by units, concerning the gender and the disease severity.
A separate application window provides an interpretation of the results of calculating the need for packages, consumption in NDDD per 100 bed-days, and the frequency of prescribing the drugs (Table 4). The obtained values are used for further pharmacoeconomic assessment.
|
Table 4: The Drugs Consumption window |
||||
|
Pharmacotherapeutic group (drug) |
Cost |
NDDD |
NDDD/100 bed-days |
The frequency of prescribing |
|
Anxiolytic and hypnotic drugs |
1,531.33 |
|
|
|
|
Anxiolytic drugs |
1,096.79 |
|
|
|
|
Clonazepam, 0.5 mg pills |
48.44 |
3 |
0.17 |
4.17 |
|
Hypnotic drugs |
214.53 |
|
|
|
|
Ivadal, 10 mg coated pills |
214.53 |
5 |
0.29 |
4.17 |
|
Hypnotic drugs and their combinations |
12,855.27 |
|
|
|
|
Antidepressant drugs |
12,855.27 |
|
|
|
|
ISRS antidepressant drugs |
10,065.42 |
|
|
|
|
Paxil, 20 mg coated pills |
1,907.10 |
90 |
5.16 |
4.17 |
|
Tricyclic antidepressants |
|
|
|
|
|
Amitriptyline, 25 mg coated pills |
221.24 |
78.5 |
4.5 |
4.17 |
|
Antipsychotic drugs |
|
|
|
|
|
Atypical antipsychotic drugs |
|
|
|
|
|
Abilify, 15 mg pills |
37,392.86 |
150 |
8.6 |
4.17 |
|
Prolonged action antipsychotic drugs |
|
|
|
|
|
Haloperidol decanoate oil solution for IM injection, 50 mg/ml, 1 ml |
503.04 |
121.2 |
6.95 |
8.33 |
|
Typical antipsychotic drugs |
6,296.93 |
|
|
|
|
Extrapyramidal Correctors |
3,280.03 |
|
|
|
|
PK-Merz, 100 mg coated pills |
195.32 |
11.5 |
0.66 |
4.17 |
|
Cyclodol, 2 mg pills |
3,084.71 |
468.8 |
26.87 |
70.83 |
The third block provides the assessment of the total cost of the drugs and the cost of each drug, which are grouped by clinical and statistical groups, by the unit, or by the gender.
The information about cost distribution by the pharmacotherapeutic groups is available for viewing in the form of tables and charts (Figure 3, Table 5).
|
Table 5: Cost distribution |
||
|
Pharmacotherapeutic group |
Cost, rubles |
Share, % |
|
Total |
1,400,324.19 |
|
|
Antipsychotic drugs |
1,206,892.49 |
86 % |
|
Atypical antipsychotic drugs |
1,037,828.88 |
including 86 % |
|
Typical antipsychotic drugs |
63,966.71 |
5 % |
|
Prolonged action antipsychotic drugs |
105,096.90 |
9 % |
|
Antidepressant drugs |
76,853.12 |
5 % |
|
Anxiolytic and hypnotic drugs |
25,382.39 |
2 % |
|
Mood stabilizing agents |
42,816.97 |
3 % |
|
Extrapyramidal correctors |
30,416.22 |
2 % |
|
Neurometabolic stimulants |
4,598.31 |
0.33 % |
|
Neurotropic drugs |
1,655.28 |
0.12 % |
|
Other drugs |
11,709.41 |
1 % |
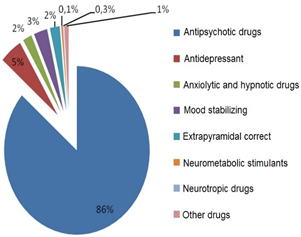
Figure 3: Cost distribution
After entering the individual data for the studied cohort of patients (n = 198) and calculating the costs, three types of pharmacoeconomic analysis become available: Cost Minimization, Cost and Efficacy, and Impact on the Budget.
The Cost Minimization analysis implies that the efficacy and safety of the studied drugs are equal. In the Cost Minimization analysis, two drug names (brand names) are compared, and the difference between the costs of the two compared drugs is calculated (Figure 4, Table 6). For instance, according to the literature, olanzapine and clozapine do not differ in efficacy from the standpoint of the exacerbations frequency; it is, therefore, possible to use the comparative Cost Minimization pharmacoeconomic analysis [15-17].
|
Table 6: Cost Minimization analysis |
||
|
Indicators |
Analogous drug |
Drug |
|
Olanzapine |
Clozapine |
|
|
Cost per patient, rubles |
5,083.2 |
1,239.6 |
|
Difference, rubles |
|
-3,843.6 |
|
Difference, % |
|
-76 |
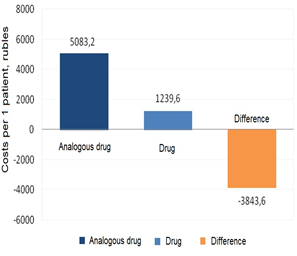
Figure 4: Performing the Cost Minimization analysis
The results of the Cost Minimization analysis showed that the cost per patient was higher with olanzapine, and the difference between the costs of the two drugs (olanzapine and clozapine) was -76 % (-3,843.60 rubles).
For the Cost and Efficacy analysis, two drugs are selected, and the name and the value (in percent) of the efficacy criterion are entered (e.g., for the patients with schizophrenia, it is advisable to use the exacerbations frequency on the background of drug treatment) [18].
For the Cost and Efficacy analysis, 56 randomized controlled studies from the literature data were analyzed [18], of which five direct studies of olanzapine and haloperidol were chosen, including the three studies performed at the inpatient stage of treatment, since for these drugs, the difference in their efficacy was statistically veracious [19-21]. The criterion of efficacy was the absence of exacerbations during therapy (Table 7).
|
Table 7: The frequency of exacerbations and the efficacy of treating the schizophrenia with haloperidol and olanzapine [18] |
|||||
|
Study |
The number of patients |
The number of exacerbations, % |
Weight (W) |
Sources |
|
|
Olanzapine (OLZ) |
Haloperidol (HAL) |
||||
|
RCS 1 |
166 |
19 |
11 |
0.1655 |
[19] |
|
[20] |
|||||
|
RCS 2 |
423 |
20 |
40 |
0.4217 |
|
|
[21] |
|||||
|
RCS 3 |
414 |
12 |
22 |
0.4128 |
|
|
Total |
1003 |
- |
- |
- |
|
|
The average number of exacerbations, % |
17 |
28 |
- |
||
|
Efficacy, % |
83 |
72 |
- |
||
For the Cost and Efficacy analysis, the data of the treatment standard for the patients with schizophrenia were used: schizophrenia in the acute (subacute) phase, with resistance, and intolerance to the therapy. For instance, the average duration of hospitalization was 60 days. The average therapeutic daily doses for olanzapine and haloperidol were 15 and 20 mg, respectively [22].
With the weighted average exacerbation rate of 17 % and 28 % for olanzapine and haloperidol, respectively, the efficacy of the therapy (the number of prevented exacerbations) was 83 % and 72 % for olanzapine and haloperidol, respectively.
The results of the pharmacoeconomic analysis are presented in the form of the cost-effectiveness ratio (CER) and incremental cost-effectiveness ratio (ICER) (Table 8).
|
Table 8: The results of the Cost and Efficacy analysis |
||
|
Indicators |
Analogous drug |
Drug |
|
Trade name |
Haloperidol, 10 mg pills |
Zalasta, 10 mg pills |
|
INN |
Haloperidol |
Olanzapine |
|
The average cost per patient, rubles |
113.45 |
5,083.20 |
|
Difference, rubles |
4,969.75 |
|
|
Ef, % |
72 |
83 |
|
Ef difference |
11 |
|
|
CER |
157.57 |
6,124.34 |
|
ICER |
45,179.50 |
|
Thus, the results of the Cost and Efficacy pharmacoeconomic analysis for individual treatment regimens show that the ICER amounted to 45,179.5 rubles.
The results of the Cost Minimization and Cost and Efficacy pharmacoeconomic analyses may be further used for the Impact on the Budget analysis. The application calculates the initial parameters of the model: the frequency of drug prescriptions and the cost of therapy for the studied cohort of patients (n = 198), after which the user sets the desired frequency of the drug prescriptions.
The Impact on the Budget analysis is based on the assumption that the average number of DDD doses per patient remains unchanged. The results of the analysis are shown in tables and graphs (Figure 5, Table 9).
|
Table 9: Results of the Impact on the Budget analysis |
|||||||
|
Indicators |
Initial data |
The Impact on the Budget |
|
|
Indicators |
Initial data |
The Impact on the Budget |
|
Drug 1 |
275,575.17 |
141,320.60 |
|
|
Inpatient care cost, rubles |
1,447,601.16 |
1,320,906.33 |
|
Drug 2 |
6,763.98 |
14,303.72 |
|
|
Difference, rubles |
|
-126,694.83 |
|
Total for the two drugs, rubles. |
282,339.15 |
115,644.32 |
|
|
Difference, % |
|
-8.8 |
|
Difference, rubles |
|
-126,694.83 |
|
|
|
|
|
|
Difference, % |
|
-45 |
|
|
|
|
|
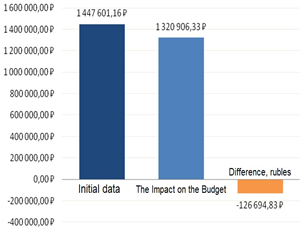
Figure 5: Results of the Impact on the Budget analysis
For instance, with increasing the frequency of prescribing the more effective and expensive olanzapine by 19 %, a decrease in the cost by 45 % (126,694.83 rubles) will be observed due to a decrease in the haloperidol prescribing frequency.
The results of the Impact on the Budget pharmacoeconomic analysis are indexed for the user-specified value (Table 10).
|
Table 10: Cost indexation of the Impact on the Budget analysis |
||
|
Price adjustment coefficient |
Inpatient care cost, rubles |
|
|
|
BEFORE |
AFTER |
|
Adjust cost by 5 % |
1,519,981.22 |
1,393,286.83 |
For instance, with an inflation rate of 5 %, the predicted budget after the analysis would amount to 1,393,286.39 rubles, and the cost of treatment would decrease by 8.3 %.
The third block of the calculator also implements the possibility of independent pharmacoeconomic analyses via entering new data per patient.
For the independent Cost Minimization analysis, the initial data about the drugs are entered: the dose per administration, the dosage frequency, and the number of the days of administration.
The application calculates the cost per patient and the difference between the costs of the two drugs. The results of the Cost Minimization analysis for the drugs with equal efficacy (clozapine and olanzapine) are shown on the chart in Figure 6 and Table 11.
|
Table 11: The results of the independent Cost Minimization pharmacoeconomic analysis |
||
|
Indicators |
Analogous drug |
Drug |
|
Cost per patient |
14,523.43 |
1,789.13 |
|
Difference, rubles |
|
-12,734.30 |
|
Difference, % |
|
-87.7 |
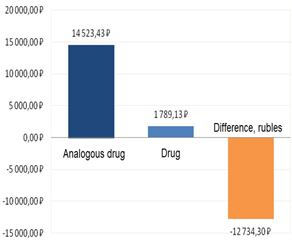
Figure 6: The results of the independent Cost Minimization pharmacoeconomic analysis
For instance, in the independent Cost Minimization analysis, the difference between the costs of olanzapine and clozapine was -87.7 % (-12,734.30 rubles).
For the Cost and Efficacy analysis, two drugs are selected, and the name and the value (in percent) of the efficacy criterion are entered (e.g., for the patients with schizophrenia, it is advisable to use the exacerbations frequency on the background of drug treatment) [18].
The weighted average efficacy of therapy (the number of exacerbations) was 72 % and 83% for haloperidol and olanzapine, respectively.
|
Table 12: The results of the independent Cost and Efficacy pharmacoeconomic analysis |
||
|
Indicators |
Analogous drug |
Drug |
|
Trade name |
Haloperidol, 10 mg pills |
Zalasta, 10 mg pills |
|
INN |
Haloperidol |
Olanzapine |
|
The average cost per patient, rubles |
163.37 |
14,523.43 |
|
Difference, rubles |
|
14,360.06 |
|
Ef, % |
72 |
83 |
|
Ef difference |
|
11 |
|
CER |
226.90 |
17,498.11 |
|
ICER |
|
130,546.01 |
The independent Cost and Efficacy analysis showed that haloperidol was less expensive, but olanzapine was more effective for preventing exacerbations; compared to haloperidol, its ICER was 130,546.01 rubles (Table 12).
The results of the Cost and Efficacy pharmacoeconomic analysis can be used for modeling the economic consequences of increasing the frequency of using a more effective drug treatment technology.
For the independent Impact on the Budget analysis, the INN and the dosage form of the drug are chosen, and the dosage per patient, the dosage frequency, the number of days of the administration, and a hypothetical cohort of patients are entered. The results of the independent pharmacoeconomic analysis are shown graphically (Figure 7, Table 13).
|
Table 13: The result of the independent Impact on the Budget pharmacoeconomic analysis |
|||
|
Indicators |
Cost per patient, rubles |
Initial data |
The Impact on the Budget |
|
Drug 1 |
416.30 |
33,303.94 |
29,140.94 |
|
Drug 2 |
14,868.51 |
1,784,221.71 |
1,932,906.86 |
|
Total |
|
1,817,525.65 |
1,962,047.80 |
|
Difference |
|
|
144,522.15 |
|
Difference, % |
|
|
7.95 |
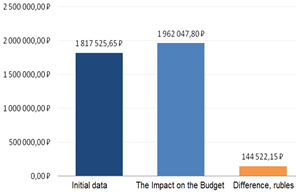
Figure 7: The result of the independent Impact on the Budget pharmacoeconomic analysis
Further, the Impact on the Budget analysis showed that increasing the frequency of prescribing the more effective and more expensive olanzapine by 5 % would increase the cost by 7.95 % due to decreasing the frequency of prescribing haloperidol (Figure 6).
Conclusions
The developed interactive software expands the possibilities of the pharmacoeconomic analysis, increases the efficiency of presenting the results, and is necessary for various health care professionals, as well as for the people engaged in planning the budget in the health care sector at various management levels, including CEOs of medical care organizations. The analytical model can be a tool of the pharmacoeconomics manager engaged in pharmacoeconomic assessment for the rational use of the economic resources of a psychiatric clinic with the use of the following methods: the Impact on the Budget analysis, the Cost and Efficacy analysis, and the Cost Minimization analysis.
Acknowledgments
Supported by the "Russian Academic Excellence Project 5-100".
References
- Sotsialno znachimye zabolevaniya naseleniya Rossii v 2018 godu: statisticheskie materially [Socially significant diseases of the population of Russia in 2018: statistical materials]. Moscow: Ministry of Health of the Russian Federation; 2019, 73 p. Available from: https://www.rosminzdrav.ru/ministry/61/22/stranitsa-979/statisticheskie-i-informatsionnye-materialy/statisticheskiy-sbornik-2018-god
- Lyubov EB, Yastrebov VS, Shevchenko LS, Chapurin SA, Churilin YuYu, Bylim IA, Gazha AK, Doronin VV, Kosov AM, Petukhov Yul, Fadeev PN. Ekonomicheskoe bremya shizofrenii [The economic burden of schizophrenia]. Social and clinical psychiatry. 2012; 3: 36–4
- Haider S, Nisar QA, Baig F, Azeem M. Dark Side of Leadership: Employees' Job Stress & Deviant Behaviors in Pharmaceutical Industry. Int. J. Pharm. Res. Allied Sci. 2018 Apr 1;7(2):125-138.
- Sergeevna SM, Efimovna LE. Improving training of pharmaceutical specialists for consulation in pharmacy organizations using interactive forms of education. Pharmacophores. 2020 Mar 1;11(2):7-1
- Federal law No 323-FZ "Ob osnovakh okhrany zdorovya grazhdan v Rossiiskoi Federatsii" [On the Bases of Health Protection of the Citizens in Russian Federation] dated 21.11.2011. Available from: https://www.rosminzdrav.ru/documents/7025
- Omelyanovsky VV. OTZ pomozhet raskhodovat sredstva ratsionalno [Assessment of health care technologies to help spending funds sparingly]. Rossiyskaya Gazeta. 2019; Special issue 65 (7823). Available from: https://rg.ru/2019/03/25/otz-pomozhet-rashodovat-sredstva-racionalno.html
- Applying Principles of Pharmacoeconomics to Improve Medical Product Selection and Use in Low- and Middle-income Countries: Trainer’s Guide. Department of Global Health. University of Washington; 2017, 100 p.
- 22. Sundus A, Ismail NE, Gnanasan S. Exploration of healthcare practitioner’s perception regarding pharmacist’s role in cancer palliative care, malaysia. Pharmacophores. 2018 Jul 1;9(4):1-7.
- GOST R 56044-2014 Evaluation of medical technologies. General regulations Intr. 2015-06-01. Moscow: Standartinform; 2015, 46 p.
- Industry Standard 91500.14.0001-2002 Clinical and economic studies. General regulations Intr. 2002-05-27. 2002.
- Yagudina RI, Kulikov AY, Serpik VG. Farmakoekonomika [Pharmacoeconomics]: textbook. Second edition. Rostov-on-Don: Phoenix; 2018, 237 p.
- Programma PharmCompile (russ.) - Avtomatizatsiya AVS/VEN/DDD-analiza [PharmCompile application (rus) - Automation of the ABC/VEN/DDD analysis]. Available from: http://clinical-pharmacy.ru/article/965-programma-farmkompayl-russ-avtomatizaciya-avsvenddd-analiza.html
- ARM klinicheskogo farmakologa [WKS of a clinical pharmacologist]. Available from: http://pharmsuite.ru/web2/
- The official website of the Russian Association of Ontological Mammology. Available from: http://www.breastcancersociety.ru/
- Komossa K, Rummel-Kluge KH, Gunger Kh, Shmid F, Shvarts S, Dugan L, Kisling V, Lyokht S. Olanzapine v sravnenii s drugimi atipichnymi antipsikhotikami pri lechenii shizofrenii [Olanzapine compared to other atypical antipsychotic drugs in the treatment of schizophrenia]. Social and clinical psychiatry. 2012; 1: 63–70.
- Lyokht S, Komossa K, Rummel-Kluge KH, Korves K, Khutger Kh, Shmid F, Lobos KA, Shvarts S, Davis JM. Meta-analiz pryamykh sravnenii antipsikhotikov vtorogo pokoleniya pri lechenii shizofrenii [Meta-analysis of direct comparisons of second-generation antipsychotic drugs in the treatment of schizophrenia]. Social and clinical psychiatry. 2011; 4: 67–71.
- Bitter I, Dossenbach MR, Brook S, Feldman PD, Metcalfe S, Gagiano CA, Füredi J, Janka Z, Kovacs G, Bartko G, Banki CM, Breier A. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004; 1: 173–180.
- Zhao YJ, Lin L, Teng M, Khoo AL, Soh LB, Furukawa TA, Baldessarini R, Lim BP, Sim K. Long-term antipsychotic treatment in schizophrenia: a systematic review and network meta-analysis of randomized controlled trials. Br J Psychiatry Open. 2016; 2(1): 59–66.
- Crespo-Facorro B, Perez-Iglesias R, Mata I, Caseiro O, Martinez-Garcia O, Pardo G, Ramirez-Bonilla M, Pelayo-Terán JM, Vázquez-Barquero JL. Relapse prevention and remission attainment in first-episode non-affective psychosis. A randomized, controlled 1-year follow-up comparison of haloperidol, risperidone, and olanzapine. Journal of psychiatric research. 2011; 45: 763–769.
- Hamilton SH, Edgell ET, Revicki DA, Breier A. Functional outcomes in schizophrenia: a comparison of olanzapine and haloperidol in a European sample. International clinical psychopharmacology. 2000; 15: 245–255.
- Keefe RS, Young CA, Rock SL, Purdon SE, Gold JM, Breier A. One-year double-blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophrenia research. 2006; 81: 1–15.
- Order of the Ministry of Health of Russia No. 1233n "Ob utverzhdenii standarta spetsializirovannoi meditsinskoi pomoshchi pri shizofrenii, ostroi (podostroi) faze, s rezistentnostyu, intolerantnostyu k terapii" [On Approving the Standard for Specialized Medical Care for schizophrenia, acute (subacute) phase, with resistance, and intolerance to therapy] dated 12.12.2012. Available from: https://legalacts.ru/doc/prikaz-minzdrava-rossii-ot-20122012-n-1233n/.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India
