Comparison of susceptibility artifact on rectal DWI-MRI and rectal volume before and after ultrasound gel versus Microlax enema
Maryam Farghadani1, Farhad Rezaei2*, Mehdi Karami3
1 Assistant Professor of Radiology, Department of Radiology, Cancer Prevention Research Center, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran, 2 Resident of Radiology, Department of Radiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran, 3Associate Professor of Radiology, Department of Radiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
Correspondence: Farhad Rezaei. Resident of Radiology, Department of Radiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
Email: farhad.rezaei.1 @ gmail.com
|
ABSTRACT Introduction: MRI is necessary for rectal cancer staging. Adding DWI sequence has various benefits. Air in rectum decreases images quality especially on DWI sequence. For this purpose, Rectal enema has been offered. Rectal enema could compress mucosa and potentially change T stage of cancer. Enema with ultrasound gel was compared with enema with Microlax according to their extent of decreased susceptibility artifact and extent of rectal distension. Material and Methods: 40 patients with known rectal cancer were enrolled. 20 patients had enema with Microlax, and 20 patients had enema with ultrasound gel. For each patient, rectal MRI before and after enema was taken, and susceptibility artifact of DWI images before and after enema and the volume inside rectal lumen before and after enema were calculated, and compared between two groups. Results: Mean Susceptibility artifact after enema with Microlax (1.77±1.47) and ultrasound gel (1.97±1.16) decreased significantly versus pre enema images ((3.325±1.35) for Microlax and (3.32±1.37) for ultrasound gel) (P value<0.001 for each group). Mean volume inside rectal lumen after enema with Microlax was (14.38±5.352 cm3), and after ultrasound gel enema was (31.33±9.494 cm3). Rectal distension with ultrasound gel enema was statistically more than enema with Microlax (P-value<0.001). Conclusion: Enema with about 60 cc of Microlax was offered before DWI MRI of rectum. This enema decreased susceptibility artifact. Furthermore, Microlax caused less rectal distension in comparison to ultrasound gel, so T-staging was more precise with Microlax versus ultrasound gel enema on MRI T2 Sequence. Keywords: Rectal cancer, Diffusion weighted (DWI) MRI sequence, susceptibility artifact, rectal distension, ultrasound gel enema, Microlax enema |
Introduction
The best modality for local staging of rectal cancer is MRI. The main sequence for staging is T2 sequence. Axial plane should be perpendicular to the rectal wall at cancer site, and coronal plane should be parallel to the anal canal [1, 2]. In recent years, adding diffusion weighted sequence (DWI) has resulted in the following benefits:
1- Increased sensitivity for detecting tumors [3-6], 2- Improved detecting of involved lymph nodes [6-8], 3- Evaluation of response to chemotherapy or radiotherapy and differentiation of fibrosis from viable tumoral tissue after treatment [5, 9-12], 4- Prediction of response to chemotherapy or radiotherapy [13] and 5- better depicting of mesorectal fascia clearance from tumor after chemotherapy or radiotherapy [14]. Susceptibility artifact on MRI images has been defined as localized distortion of image signal or signal loss in areas where magnetization is changed significantly. Susceptibility artifact is mostly seen at air-tissue, bone-tissue, and metal-tissue interfaces [15]. The main artifact on rectal DWI sequence is susceptibility artifact due to air in rectum, that in 16 percent of patients it could make imaging uninterpretable [10]. To decrease this artifact, two solutions have been present, first is the alteration of DWI parameters and the second one is the elimination of the cause of artifact (air in rectum) [10]. Many studies have worked on changing the parameters of DWI sequence such as decreased field of view [16], parallel imaging or bipolar DWI imaging [17, 18]. Some studies have focused on replacing rectal air by enema. In almost all of these studies, enema by ultrasound gel has been done [1, 10]. Rectal distension could compress the rectal mucosa, and change the distance between tumor and mesorectal fascia [19]. A new research published on 2018 concluded that endorectal filling with ultrasound gel before MRI did not result in improved T-staging, and could falsely increase T-stage of tumor due to the pressure effect on mesorectal fat [20]. In this study and some other studies, it was concluded that MRI without enema led to more precise T-staging [20, 21]. In one study, it was offered that enema with 5 cc Microlax could significantly decrease susceptibility artifact on DWI sequence without distension of rectum [10]. We designed a study to compare the change of susceptibility artifact on DWI sequence after ultrasound gel enema with this change after enema with Microlax. Also, the extent of rectal distension after enema with ultrasound gel was compared with this distension after enema with Microlax. After these comparisons, the authors wanted to know which enema would result in better DWI images and lesser extension of rectum.
Material and Methods:
Ethics: Each patient was informed about the whole process of study and signed the consent form.
Also, this study did not have any side effects on patients.
Study design:
The study started in January 2018 and ended in January 2019. We collected 40 patients with known rectal cancer were the participants. All patients referred to the MRI center for primary staging or restaging (after treatment). The MRI machine was 1.5 Tesla, Ingenia, Philips (model of Omega).
The patients were randomly divided into two groups: group 1: 20 patients who underwent enema with Microlax, and group 2: 20 patients who underwent enema with ultrasound gel.
For each patient before applying enema T2-weighted sequence, T1-weighted sequence without and with Gadolinium IV injection, DWI and ADC map were acquired (each patient had GFR more than 60 cc/min/1.73 m2 and was suitable for IV injection of Gadolinium). After enema, only T2 and DWI sequences and ADC map were obtained. Parameters of MRI sequences after enema were the same as the counterpart sequences before enema. For DWI sequence, b-value was 1000 s/mm2. The thickness of each axial slice was 4 mm for DWI sequence.
For 20 patients, enema with Microlax, and for other 20 patients enema with ultrasound gel was done. MICROLAX® is a micro-enema which softens the stool for treatment of constipation. The amount of ultrasound gel or Microlax used for enema was 60 cc. Enema was applied 5 to 10 minutes before MRI acquisition. The compliance of patients for applying 60 cc enema was perfect, and no patient rejected receiving enema.
Ultrasound gel enema was compared versus Microlax enema regarding their effect on two variables: 1) susceptibility artifact on DWI sequence and 2) rectal distension. For susceptibility artifact, two radiologists with expertise in interpretation of pelvic MRI subjectively gave a score to DWI images of each patient before enema and a score for after enema DWI images. The two radiologists agreed on a consensus for subjective scoring system used 6 scores to depict the extent of susceptibility artifact on DWI images (0 for no artifact, 1 for mild, 2 for mild to moderate, 3 for moderate, 4 for moderate to severe and 5 for severe artifact) (Figure 3). Each patient was given two scores by each radiologist, one score for DWI images before enema, and another score for after enema. The averages of two scores for before enema images and two scores for after enema images given by two radiologists for each patient were calculated, and the final scores were considered for data analysis.
Regarding rectal distension by enema, the volume inside rectal lumen was calculated between upper and lower borders of cancer. On axial images of T2 sequence, from lower up to upper borders of cancer , the area of lumen at each slice was calculated (by the software of MRI workstation), then these numbers were summed, and the summed number was multiplied by 0.15 cm (thickness of each slice). The final number was the approximate volume inside rectal lumen in unit of cm 3. For each patient, this volume was calculated before and after the enema.
The authors compared the susceptibility artifact on DWI sequence between: 1- patients in group 1 before enema with patients in group 2 before enema, 2- images before enema with images after enema in patients of group 1, 3- images before enema with images after enema in patients of group 2; and the patients compared the extent of decreased susceptibility artifact after enema (in comparison to before enema images) between patients of group 1 and 2.
Score equal to or more than 3 (for DWI susceptibility artifact) was considered clinically important because of hampering correct interpretation of images.
Also, the numbers of score 3 or more on DWI images before and after the enema were compared between group 1 and group 2.
In addition, the volumes inside rectal lumen between the upper and lower parts of cancer before the enema between patients of group 1 and 2 were compared, then this volume was compared before the enema with this volume after the enema for each group. Lastly, the extent of increased volume of this space after enema (in comparison to before enema images) was compared between the patients of group 1 and 2.
Data analysis:
Statistical analysis was done by IBM SPSS version 22 software. For data analysis Chi-Square test (for comparison between nominal and qualitative data between two groups), T-test (for Comparison between quantitative data between two groups) and T-paired test (for comparison between quantitative data before and after enema in each group) were used.
Results:
Mean susceptibility artifact before enema for patients of group 1 was (2.7±1.35), and for patients of group 2 was (3.32±1.38). Mean susceptibility artifact after enema for patients of group 1 and group 2 was (1.77±1.47) and (1.97±1.16); respectively (Figure 1).
Mean volume of space in rectal lumen between the upper and lower parts of cancer before enema for patients of group 1 was (5.39±1.427 cm3), and for patients of group 2 before enema was (5.28±0.891 cm3). This volume after enema for patients of group 1 and 2 was (14.38±5.352 cm3) and (31.33±9.494 cm3); respectively (Figure 2).
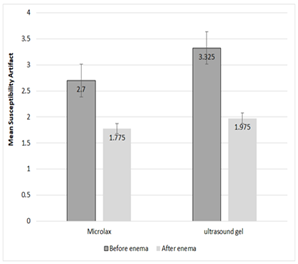
Figure 1. Mean Susceptibility artifact before and after enema with Microlax and ultrasound gel
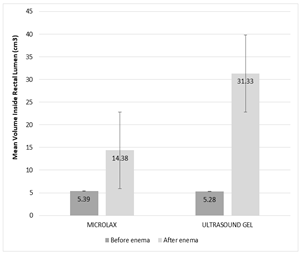
Figure 2. Mean Volume inside rectal lumen before and after enema with Microlax and ultrasound gel
Mean susceptibility artifact before enema for patients of group 1 was the same as this artifact before enema for group 2 patients (P-value: 0.156). This artifact after enema with Microlax (1.77±1.47) and ultrasound gel (1.97±1.16) significantly decreased versus before enema images of Microlax (3.325±1.35) and ultrasound gel group (3.32±1.37) ; respectively (P-value<0.001 for each group) (Figure 4). Mean susceptibility artifact after enema with Microlax was the same as this artifact after enema with ultrasound gel (P-value: 0.637). The decrease of clinically important susceptibility artifact numbers after Microlax enema in group 1 (versus before enema images) was the same as this decrease after ultrasound gel enema in group 2 (versus before enema images) (P-value: 0.082)
Mean volume inside rectal lumen between upper and lower borders of cancer before microlax enema in group 1 was the same as this volume before ultrasound gel enema in group 2 (P-value: 0.771). This volume increased significantly after enema with Microlax (14.38±5.352 cm3) and ultrasound gel (31.33±9.494 cm3) versus before enema volume for microlax group (5.39±1.427 cm3) and ultrasound gel group (5.28±0.891 cm3); respectively (P-value<0.001 for each group). Increase of mean volume inside rectal lumen after ultrasound gel was more than Microlax enema (in comparison to before enema volumes) (P-value<0.001).
Lastly, the authors considered the effect of four potential confounders: Age, gender, BMI and existence or absence of constipation on susceptibility artifact and volume inside rectal lumen. All four confounders had no effect on these variables in each group or between two groups.
|
|
|
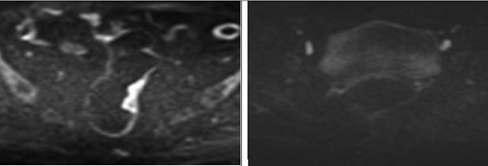
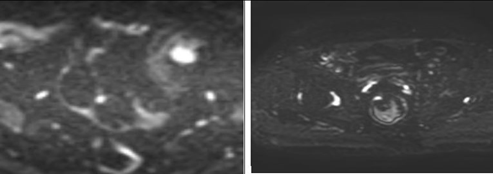
Figure 3a. Susceptibility artifact score on DWI: Score 0 (left) and 1 (right) |
|
|
|
Figure 3b. Susceptibility artifact score on DWI: Score 2 (left) and 3 (right) |
|
|
|
Figure 3c. Susceptibility artifact score on DWI: Score 4 (left) and 5 (right) |
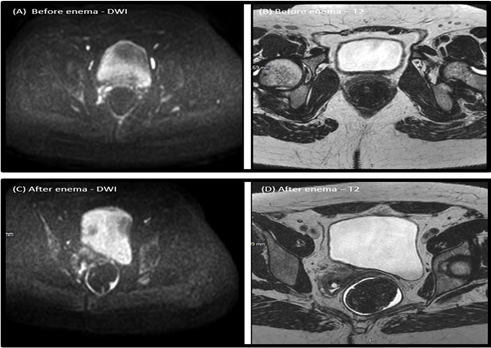
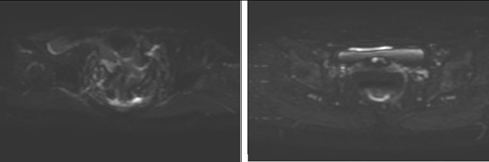
Figure 4. DWI and T2 sequences before enema (A and B) and after enema (C and D)
Discussion:
MRI is used more for rectal cancer staging, and DWI sequence is becoming a part of routine sequences. Different methods have been applied to improve the quality of DWI images [8]. Many studies have worked on changing the parameters of DWI sequence such as decreased field of view [16], parallel imaging [17] or bipolar DWI imaging [18]. Some studies have shown that rectal enema could improve the quality of DWI images. In almost all studies on enema, the substance for enema was ultrasound gel, and the amount of enema was 50 to 150 cc [10]. A study by Slater et al., (2006) showed that the distance of outer rectal wall to the mesorectal fascia decreased after 100 cc room air insufflation to the rectum [19]. The extent of cancer invasion beyond muscularis propria has been determinative for rectal staging [22]. Also, the distance between tumor and mesorectal fascia has been crucial for cancer prognosis [23]. If the distance between tumor and mesorectal fascia (MRF) is more than 1 mm, MRF is considered clear from cancer, and this is suggestive of negative margins after TME (total mesorectal excision) [24]. One study has shown that endorectal filling could lead to overstaging of rectal cancer on T2 sequence due to pressure effect on mesorectal fat [20]. To overcome this obstacle, Griethuysen et al. (2018) used micro enema with 5 cc Microlax before DWI sequence, and their results showed the decrease of susceptibility artifact after micro enema versus without enema DWI images [10]. In this study, the authors applied 60 cc enema for each patient. In both groups, (Microlax and ultrasound gel) susceptibility artifact decreased significantly. When the susceptibility artifact after enema was compared between two groups, there was no statistical difference. Therefore, the authors concluded that the decrease of susceptibility artifact by Microlax was the same as ultrasound gel enema. Because the authors applied 60 cc enema, enema material in all slices of MRI images was seen. Therefore, the susceptibility artifact on DWI sequence in all slices was decreased. In the study done by Griethuysen et al. (2018), the amount of Microlax enema was 5 cc [10]. The authors thought 5 cc enema was too little specially for cancers lying at high part of rectum, and 5 cc could not decrease the susceptibility artifact in all slices. Also, the authors found that the volume inside rectal lumen after enema was increased significantly in comparison to this volume before enema either by Microlax or by ultrasound gel. Increased volume after enema with Ultrasound gel was more than Microlax. As mentioned before the enema material could alter the distance between tumor and mesorectal fascia and compress mesorectal fat, and therefore could change T-stage [19, 20].
Conclusion:
The authors would offer enema with about 60 cc Microlax before DWI sequence of rectal MRI because this decreased the susceptibility artifact on DWI and other sequences, and because the rectal distension caused by Microlax was less than Ultrasound gel, so T-staging with Microlax was more precise.
Limitations:
One of the limitations was that for every patient 60 cc enema was used. The authors thought that if a similar study would be done using different amounts of enema according to the location of cancer (high, middle or low in rectum), the results would be more precise. In addition, if a study would be designed to compare rectal staging based on MRI after Microlax enema with staging based on pathology, the authors could decide better whether to use Microlax enema before MRI or not.
Financial Support:
Research Department of Isfahan University of Medical Sciences supported this study financially.
Acknowledgement:
The authors would like to thank Research Department of Isfahan University of Medical Sciences for their financial support, and also thank MRI department of Al-Zahra Hospital (The main hospital of Isfahan University of Medical Sciences) for their cooperation regarding the admission of the patients of this study.
Conflict of Interest:
There were not any conflicts of interest between the authors.
Authors’ Contributions:
MF contributed in the conception of study, collecting patients, reporting all MRI images, analysis of MRI images, approving final version of manuscript and MF agreed with all parts of final manuscript.
FR contributed in gathering patients, analysis of MRI images, approval of final version of manuscript, and FR agreed with all parts of the final manuscript.
MK contributed in drafting the study and revising the final version of manuscript, and MK agreed with all parts of the final manuscript.
References
- S. Palmucci, M. Piccoli, et al., Diffusion MRI for rectal cancer staging: ADC measurements before and after ultrasonographic gel lumen distension, European Journal of Radiology,86 (2017) 119–126
- Courtney C. Moreno, Patrick S. Sullivan, Pardeep K. Mittal, MRI Evaluation of Rectal Cancer: Staging and Restaging, Current Problems in Diagnostic Radiology 46 (2017) 234–241
- Li F1, Zhang W, Li J, Zhu X, Chen H, et al., The clinical application value of MR diffusion-weighted imaging in the diagnosis of rectal cancer: A retrospective study, Medicine (Baltimore). 2018 Dec 97(51): e13732
- Hosonuma T, Tozaki M, Ichiba N, et al., Clinical usefulness of diffusion-weighted imaging using low and high b-values to detect rectal cancer. Magnetic resonance in medical sciences, 2006; 5 (4): 173–7.
- Supreeta Arya, Deepak Das, Reena Engineer, and Avanish Saklani. Imaging in rectal cancer with emphasis on local staging with MRI. Indian J Radiol Imaging. 2015 Apr-Jun; 25(2): 148–161
- Kartik S. Jhaveri and Hooman Hosseini-Nik .MRI of Rectal Cancer: An Overview and Update on Recent Advances. American Journal of Roentgenology. 2015;205 (1): W42-W55.
- Mir N, Sohaib S, Colins D, Koh DM, Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis, J Med Imaging Radiat Oncol 2010; 54: 358–64.
- Qili zhao, Lijian liu, et al., Preoperative diagnosis and staging of rectal cancer using diffusion-weighted and water imaging combined with dynamic contrast-enhanced scanning, Oncol Lett. 2014 Dec; 8(6): 2734–2740.
- Eisenhauer EA, Therasse P, et al., New response evaluation criteria in solid tumours: revised RECIST guide-line (version 1.1), Eur J Cancer 2009; 45(2): 228–47
- J. J. M. van Griethuysen, E. M. Bus, M. Hauptmann, L. Molenaar, et al., Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T – Effect of applying a micro-enema to improve image quality, European Journal of Radiology 99 (2018) 131–137
- S.H. Kim, J.M. Lee, S.H. Hong, et al., .2009. Locally advanced rectal cancer: Added value of diffusion-weighted MRI in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy, Radiology, 253 (1), pp. 116-125
- D.M.J. Lambregts, V. Vandecaveye, B. Barbaro, et al.,.2011. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: A multicenter study, Ann Surg Oncol, 18 (8), pp. 2224-2231
- Dzik-Jurasz A, Domenig C, George M et al., Diffusion MRI for prediction of response of rectal cancer to chemo-radiation, Lancet 2002; 360 (9329), pp: 307–8
- Min Jung Park, Seong Hyun Kim, Soon Jin Lee, et al., Locally Advanced Rectal Cancer: Added Value of Diffusion-weighted MR Imaging for Predicting Tumor Clearance of the Mesorectal Fascia after Neoadjuvant Chemotherapy and Radiation Therapy, Radiology. 2011 Sep; 260(3):771-80
- Susie Y. Huang, Ravi T. Seethamraju, et al., Body MR Imaging: Artifacts, k-Space, and Solutions, RadioGraphics Volume 35, Issue 5, Jul 24 2015, pp: 1439-1624.
- N. Korn, J. Kurhanewicz, S. Banerjee, et al., Reduced-FOV excitation decreases susceptibility artifact in diffusion-weighted MRI with endorectal coil for prostate cancer detection, Magnetic resonance imaging, 33 (2015), 56–62
- K. Nasu, Y. Kuroki, S. Kuroki, et al., Diffusion weighted single shot echo planar imaging of colorectal cancer using a sensitivity encoding technique, Jpn. J. Clin. Oncol. 34 (10), (2004) 620–626
- S. Kyriazi, M. Blackledge, et al., Optimising diffusion weighted imaging in the abdomen and pelvis: comparison of image quality between monopolar and bipolar single-shot spin-echo echo-planar sequences, Eur. Radiol. 20 (10), (2010) 2422–2431.
- Slater A, Halligan S, Taylor SA, Marshall M. Distance between the rectal wall and mesorectal fascia measured by MRI: Effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol 2006; 61 (1): 65–70.
- Rutger CH Stijns, Tom WJ Scheenen, et al., The influence of endorectal filling on rectal cancer staging with MRI, British journal of radiology, 2018, Volume 91, Issue 1089, 20180205. 10.1259/bjr.20180205.
- R.G. Beets-Tan, D.M. Lambregts, M. Maas, et al., Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting, Eur. Radiol. 23 (9), (2013) 2522–2531.
- Gulgun Engin, Rasul Sharifov, Magnetic resonance imaging for diagnosis and neoadjuvant treatment evaluation in locally advanced rectal cancer: A pictorial review, World J Clin Oncol. Jun 10, 2017; 8(3): 214-229
- Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, et al., Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89 (3), pp:327-334.
- Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, St Rose S, Sebag-Montefiore DJ, Tekkis P, Brown G. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98 (6), pp:872-879.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India