Assessment of thyroid gland function by evaluating of TSH, FT3 and FT4 hormones in untreated cancer patients
Mazen Almehmadi1*, Khalid Alzahrani1, Magdi M.Salih1, Abdulaziz Alsharif1, Naif Alsiwiehri1, Alaa Shafie1, Abdulraheem A. Almalki1, Haytham Dahlawi1, Ayman Al-hazmi1, Ashwaq Al-khalidi1, Njood Al-ghoraibi1, Wedad Al-osaimi1, Ashwaq M Altahli2, Mustafa Halawi3, Abdulrhman M. Almehmadi4
1 Faculty of Applied Medical Sciences, Taif University, Taif city, Saudi Arabia. 2 Ministry of health, Taif city, Saudi Arabia. 3 Faculty of Applied Medical Sciences, Jizan University, Jizan city, Saudi Arabia. 4 Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia.
ABSTRACT
Thyroid-function-test is a routine laboratory exam performed in many patients; the test usually includes TSH, free-T3 (FT3), and free-T4 (FT4). Levels of these hormones can provide essential reading to evaluate thyroid gland functions. 92 patients were recruited into this study, mostly females diagnosed either with female-genital tract cancer or breast cancer. Levels of TSH, FT3, and FT4 were evaluated by ROCHE COBAS ® platform e501; TSH normal levels are 0.51 to 4.1 μIU/ml, FT3 between 3.6 to 6.9 pmol\L, and FT4 between 12.3 to 20.2 pmol\L. The results of this study indicate different levels of TSH, FT3, and FT4 from most cancer patients, and hypothyroidism in gastrointestinal tract cancer patients. Also, premenopausal females with cancer have shown signs of hypothyroidism. To conclude, normal levels of TSH, FT3, and FT4 were detected in most of this study patients, hypothyroidism was detected in the gastrointestinal tract and part of pre and post-menopause female cancer patients.
Keywords: Hypothyroidism; Menopause; Thyroid Stimulating Hormone; Cancer, Breast Cancer, T3 Triiodothyronine, T4 Thyroxine
Introduction
The thyroid gland is a vital gland in our body that regulates many body functions by secreting hormones [1-4]. The thyroid gland is a butterfly-like large ductless gland located in the anterior of the neck, inferior to the thyroid cartilage. This gland releases two hormones; the first released by follicular cells and affects most of the body cells and induce metabolism, it is called thyroid hormone T3 triiodothyronine and T4 thyroxine. The other part of this gland regulates calcium levels in the blood and release calcitonin hormone by parafollicular cells, which suppresses osteoclasts to stop the process of bones breaking down to compensate for calcium reduction [5, 6]. Thyroid-stimulating hormone (TSH) or thyrotropin is an important regulator of these hormones [6, 7]. TSH is produced by the pituitary gland and induces the secretion of T4 and T3. De-iodination of T4 converts it to T3 which both regulate TSH in the circulation by a negative feedback mechanism that reduces the level of TSH secretion [5, 6, 8]. Thyroid diseases are popular and affect the rate of metabolism in the human body [8]. Cancerous cell proliferation is directed also by thyroid hormones via surface receptors [9] which are also targeted for cancer therapy [10]. T3 and T4 hormones have essential roles in several immune systems functions, including releasing cytokines and inducing response [11, 12].
Thyroid-function-test is a routine laboratory exam. The aim of this test is to evaluate the thyroid gland functions capabilities, and the test results can provide an initial alert to indicate future health complications. The test usually includes TSH, free-T3 (FT3), and free-T4 (FT4). High levels of TSH and low levels of FT4 and FT3 indicate primary hypothyroidism, this is an evidence that thyroid gland is underactive. In other hand, low levels of TSH and high levels of FT4 indicate hyperthyroidism. This is an evidence that thyroid gland is overactive. Furthermore, normal range of FT4 with a little increase of TSH levels indicate subclinical hypothyroidism. When TSH is low, high levels of both FT3 and FT4 indicate primary type hyperthyroidism. Moreover, normal levels or slightly high TSH and elevation of both FT3 and FT4 are secondary hyperthyroidism.
Studies of thyroid gland status during cancer have indicated several results. In breast cancer patients both hyperthyroidism [13-15] and hyperthyroidism [15] were detected. Moreover, in colorectal cancer, pancreatic cancer, esophageal cancer, central nervous system cancer, ovarian cancer [15-19], uterine cancer, and renal cancer [20-22] hyperthyroidism was reported.
This study aimed to evaluate levels of TSH, FT3, and FT4 in untreated cancer patients. Results can help to evaluate thyroid-gland condition in these patients. We compared the percentage of normal levels of those hormones to optimal conditions. Also, we aimed to evaluate levels of TSH, FT3, and FT4 according to menopause status in female cancer patients.
Materials and Methods
Study design
This retrospective cross-sectional study was approved by the directorate of health affairs in Taif city, from 2019 to the end of 2020. All recruits in this study have to meet the following strict inclusion criteria to be included, firstly; diagnosed with any type of cancer at King Faisal Hospital (KFH) between 2019 and 2020; secondly; thyroid-function tests were performed at the time they have been diagnosed with cancer. The number of cases in KFH was more than 300; only 92 patients have satisfied this study strict inclusion criteria. TSH normal levels are 0.51 to 4.1 μIU/ml, FT3 between 3.6 to 6.9 pmol\L, and FT4 between 12.3 to 20.2 pmol\L.
Sample analysis
When patients were requested to provide biopsy samples according to physician request, a minimum of 3 mL of venomous blood was collected into a plain tube and loaded into ROCHE COBAS ® platform e501. Patients were advised to fast for 10 hours prior to collecting the blood. The sample was used to diagnose other tests including levels of TSH, FT3, and FT4. This
study collected the following information: sex, age of the patient, type of diagnosed cancer, levels of TSH, FT3, and FT4.
Statistical analysis
Microsoft excel for office was used for sorting of data, calculating frequencies, percentage, chi-square analysis, and standard deviation. The results when P-value < 0.05 were considered significant.
Results
Demographic analysis
This study included 92 cases of cancer patients who have not received any type of anti-cancer therapy so far (Table 1) and satisfied the strict inclusion criteria. The number of female cases was 62 and constituted 67.4% of the study group, and the number of male cases was 30 and constituted 32.6% of the study group.
|
Table 1: Demographic analysis of the study including sex, frequency, and age. |
||||
|
|
Number of cases |
|||
|
Sex |
|
Male |
30 |
|
|
|
Female |
62 |
||
|
Age |
≤39 |
Male |
6 |
|
|
|
|
Female |
9 |
|
|
|
40-64 |
Male |
13 |
|
|
|
|
Female |
40 |
|
|
|
≥65 |
Male |
11 |
|
|
|
|
Female |
13 |
|
|
Total |
|
92 |
||
Types of cancer
After sorting the data of this study, the categories of cancer types were arranged as follows; breast cancer, respiratory tract cancer, head and neck cancer, gastrointestinal tract cancer, urinary tract cancer, blood tumor, and female genital tract cancer. The frequencies of these types are illustrated in table 2.
|
Table 2: Cancer types are organized according to gender and tissue to show their frequencies and percentages. |
|||||
|
Sex |
Tissue |
Diagnosis |
Number of cases |
Percentage |
Total |
|
Male |
Gastrointestinal tract |
Sigmoid |
8 |
26.6 |
30 (100%) |
|
Colon mass |
2 |
6.66 |
|
||
|
Ascending colon |
4 |
13.3 |
|
||
|
Gastric mass |
2 |
6.66 |
|
||
|
Rectal mass |
2 |
6.66 |
|
||
|
Blood tumor |
Lymphoma |
2 |
6.66 |
|
|
|
Nodular Sclerosis |
2 |
6.66 |
|
||
|
Urinary tract cancer |
Bladder tumor- Invasive Carcinoma |
4 |
13.3 |
|
|
|
Skin cancer |
Squamous cell carcinoma |
4 |
13.3 |
|
|
|
Female |
Breast cancer |
Invasive Duct Carcinoma |
16 |
25.8 |
62 (100%) |
|
Invasive Lobular Carcinoma |
2 |
3.225 |
|
||
|
Invasive micropapillary carcinoma |
1 |
1.612 |
|
||
|
Female Genital tract |
Endometrial cancer |
15 |
24.19 |
|
|
|
Cervical cancer |
7 |
11.29 |
|
||
|
Anterior vaginal wall |
1 |
1.612 |
|
||
|
Uterus, cervix, and fallopian tube |
1 |
1.612 |
|
||
|
Ovarian cyst |
1 |
1.612 |
|
||
|
Uterine carcinoma |
1 |
1.612 |
|
||
|
Head and Neck cancer |
Thyroid |
5 |
8.064 |
|
|
|
Hurthle cell adenoma |
1 |
1.612 |
|
||
|
Blood tumor |
Blood tumor |
2 |
3.225 |
|
|
|
Hodgkin`s lymphoma |
1 |
1.612 |
|
||
|
Gastrointestinal tract |
Sigmoid mass |
2 |
3.225 |
|
|
|
Rectum mass |
2 |
3.225 |
|
||
|
Esophageal |
1 |
1.612 |
|
||
|
Duodenal mass |
2 |
3.225 |
|
||
|
Tubulovillous Adenoma |
1 |
1.612 |
|
||
|
Total |
92 (100%) |
|
|||
Evaluation of TSH, FT3, and FT4 levels:
Thyroid-function-tests were evaluated in different types of cancer against several factors. The results were compared to sex, age groups, type of cancer, and finally in female patients according to their menopause status (table 3). The patients who had normal TSH levels and had shown significant P-value (< 0.05) are as follows; according to sex 73.34% of male patients, 60.8% of female patients; according to age groups 57.44% of 40 to 64 years, and 62.5% of ≥65 years; according to cancer type, 100% of blood tumor patients, 68.5% of breast cancer patients, 50% of female genital tract patients, 100% of urinary tract cancer patients, and 66.67% of head and neck cancer patients; according to menopause status 61.12% of post-menopause female patients, and 56.8% of pre-menopause patients. Only gastrointestinal tract cancer group showed high levels of TSH with 59.4% of patients. For FT3 test, no patients showed high elevation of serum levels. The patients who had normal FT3 levels and showed significant P value are as follows; by sex 93.3% of male patients, and 91.17% of female patients; according to age groups 100% of ≤39 years patients, 90.25% of 40 to 64 age groups, and 86.7% of ≥65 years; according to cancer type 94.11% of breast cancer patients, 95.5% of female genital tract patients, 93.3% of gastrointestinal tract cancer patients, 100% of urinary tract cancer patients and head and neck cancer patients; according to menopause status 100% of post-menopause female patients, and 86.12%% of pre-menopause patients. The patients who had normal FT4 levels and showed significant P-value are as follows; 46.15% of male patients, and 73.6% of female patients; according to age groups, 75% of ≤39 years patients, 76.2% of 40 to 64 age groups, and 75% of ≥65 years; according to cancer type, 77.7% of breast cancer patients, 76% of female genital tract patients, 51.42% of gastrointestinal tract cancer patients, 100% of urinary tract cancer patients, and 83.33% of head and neck cancer patients; according to menopause status, 63.15% of post-menopause female patients, and 80.48% of pre-menopause patients.
|
Table 3: Evaluation of serum levels of TSH, FT3, FT4 between all patients (BT; Blood tumor, BC; Breast cancer, FGT; Female genital tract, GIT; gastrointestinal tract cancer, UTC; Urinary tract cancer, H&N; head and neck cancer, P. V; P-value; S, significant; N, non-significant). |
||||||||||||||||
|
Characteristics |
TSH |
FT3 |
FT4 |
|||||||||||||
|
Mean |
↓ |
Normal |
↑ |
P. V |
Mean |
↓ |
Normal |
↑ |
P. V |
Mean |
↓ |
Normal |
↑ |
P. V |
||
|
Sex |
Male |
3.13±2.7 |
0 |
22 (73.34%) |
8 (26.66%) |
S |
4.4±0.51 |
2 (6.67%) |
28 (93.3%) |
0 |
S |
14.06±2.6 |
12 (30.7%) |
18 (46.15%) |
9 (23.07%) |
S |
|
Female |
5.8±13.07 |
2 (2.7%) |
45 (60.8%) |
27 (36.48%) |
S |
4.3±0.7 |
6 (8.8%) |
62 (91.17%) |
0 |
S |
14.5±2.9 |
17 (23.6%) |
53 (73.6% |
2 (2.7%) |
S |
|
|
Age groups |
≤39 |
2.89±2.9 |
1 (9.09%) |
7 (63.63%) |
3 (27.27%) |
N |
4.38±4.38 |
0 |
12 (100%) |
0 |
S |
14.6±2.9 |
3 (25%) |
9 (75%) |
0 |
S |
|
40-64 |
5.27±5.27 |
2 (4.25%) |
27 (57.44%) |
18 (38.29%) |
S |
4.29±4.29 |
4 (9.75%) |
37 (90.25%) |
0 |
S |
14.39±2.86 |
10 (23.8%) |
32 (76.2%) |
0 |
S |
|
|
≥65 |
3.98±3.9 |
0 |
10 (62.5%) |
6 (37.5%) |
S |
4.45±0.99 |
2 (13.3%) |
13 (86.7%) |
0 |
S |
14.73±2.69 |
4 (25%) |
12 (75%) |
0 |
S |
|
|
Types of cancer |
BT |
2.15±0.57 |
0 |
7 (100%) |
0 |
S |
3.67±4.16 |
2 (28.5%) |
5 (71.42%) |
0 |
N |
12.76±1.76 |
4 (57.14%) |
3 (42.85%) |
0 |
N |
|
BC |
3.15±1.84 |
0 |
13 (68.5%) |
6 (31.5%) |
S |
4.4±0.47 |
1 (5.88%) |
16 (94.11%) |
0 |
S |
14.29±2.4 |
4 (22.22%) |
14 (77.78%) |
0 |
S |
|
|
FGT |
5.34±0.68 |
2 (7.6%) |
13 (50%) |
11 (43.3%) |
S |
4.49±0.68 |
1 (4.5%) |
21 (95.5%) |
0 |
S |
15.25±3.34 |
4 (16%) |
19 (76%) |
2 (8%) |
S |
|
|
GIT |
4.21±3.02 |
0 |
13 (40.6%) |
19 (59.4%) |
S |
4.36±0.73 |
2 (7.6%) |
24 (93.3%) |
0 |
S |
14.33±2.75 |
8 (22.85%) |
18 (51.42%) |
9 (25.71%) |
N |
|
|
UTC |
2.185±0.04 |
0 |
4 (100%) |
0 |
S |
4.42±0.08 |
0 |
4 (100%) |
0 |
S |
15.376±0.3 |
0 |
4 (100%) |
0 |
S |
|
|
H&N |
3.755±0.38 |
0 |
4 (66.67%) |
2 (33.34%) |
N |
4.64±0.38 |
0 |
6 (100%) |
0 |
S |
14.1±2.5 |
1 (16.6%) |
5 (83.33%) |
0 |
S |
|
|
Menopa-use |
Post |
3.05±2.38 |
2 (11%) |
11 (61.12%) |
5 (27.78%) |
S |
4.41±0.32 |
0 |
19 (100%) |
0 |
S |
15.44±3.5 |
5 (26.3%) |
12 (63.15%) |
2 (10.55%) |
S |
|
Pre |
4.87±5.66 |
0 |
25 (56.8%) |
19 (43.18%) |
S |
4.28±0.583 |
6 (16.2%) |
31 (83.8%) |
0 |
S |
14.31±2.16 |
8 (19.5%) |
33 (80.5%) |
0 |
S |
|
Percentage of optimal levels:
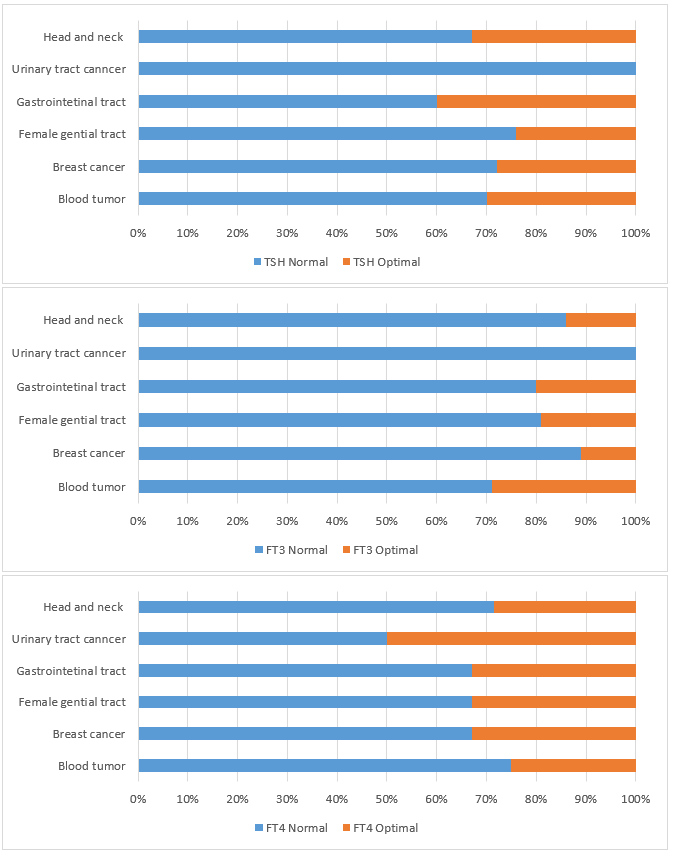
Optimal levels of patients were calculated out from the total number of patients with normal levels. Optimal levels for TSH is 0.51 to 2 μIU/ml, for FT3 is 5 to 6.9 pmol\L, and for FT4 is 15 to 20.2 pmol\L (figure 1). For TSH, the percentage of patients showed optimal levels are 32% of head and neck patients, 40% of gastrointestinal tract patients, 24% of female genital tract patients, 27% of breast cancer patients, and 30% of blood tumor patients. For FT3, the percentage of patients showed optimal levels are 20% of head and neck patients, 20% of gastrointestinal tract patients, 18% of female genital tract patients, 12% of breast cancer patients, and 28% of blood tumor patients. For FT4, the percentage of patients showed optimal levels are 28% of head and neck patients, 50% of urinary tract cancer patients, 32% of gastrointestinal tract patients, 32% of female genital tract patients, 32% of breast cancer patients and 25% of blood tumor patients.
|
|
|
|
|
|
|
Figure 1: Evaluation of percentage of patetitns with normal levels of TSH, FT3, and FT4 in cancer patietns compared to patietns with optimal levels of these hormones. |
Discussion and Conclusion
This study aimed to investigate the effect of different types of cancer on TSH, FT3, and FT4 among untreated cancer patients. 92 patients were recruited into this study, including 30 males and 62 females. Most of the study patients were females. Moreover, the highest percentage of male patients were gastrointestinal tract cancer patients, and for female patients, the highest were female genital tract patients followed by breast cancer patients. TSH can be produced by cells other than the pituitary gland, like cells involved in the immune response like T cells, B cells, and other lymphocytes [23] which are essential in combating tumor and even oncogenic virus-like human papillomavirus (HPV) [24]. Nevertheless, a study has stated that viral infection can stimulate TSH production in extra-pituitary tissues [25], therefore, assessing the levels of those hormones and correlate them with the type of cancer is important especially if this cancer is developed due to an infection. Our study has shown the normal level of TSH, FT3, and FT4 in blood tumor patients which is inconsistent with a study that detected hypothyroidism in lymphoma patients [26]. However, other studies have detected hyperthyroidism in blood tumors patients [27, 28]. Breast cancer patients have shown normal levels of TSH, FT3, and FT4, however, a small percentage of our study group showed hypothyroidism which was found in several other studies [13]. Female genital tract cancer patients mostly had normal TSH, FT3, and FT4 levels. Only a small part of the patients showed hypothyroidism which was also detected on other studies that detected hypothyroidism in uterine cancer patients [29]. Hypothyroidism is detected by our study on gastrointestinal tract cancer patients which is inconsistent with a study that detected hyperthyroidism [30]. Urinary tract patients had also normal values inconsistent with a study that detected hypothyroidism [31], however, our study group number is small. Head and neck patients had normal values with a third of the patients having hypothyroidism which is consistent with other studies reported the same results [32-34].
Pre-menopausal females with cancer have shown signs of hypothyroidism indicating signs of under-activity of the thyroid gland. This can be a risk sign of their bodies starting developing cancer. Thyroid dysfunction is detected mostly with post-menopausal females which is consistent with our study [35]. Also, hypothyroidism was reported with females that developed breast cancer [36]. To evaluate the percentage of cancer patients with optimal thyroid function by evaluating TSH, FT3, and FT4, our study measured the percentage of those patients. The highest percentage of those patients was detected in gastrointestinal tract patients, followed by head and neck patients.
Overall, our study evaluated thyroid gland activity in different untreated cancer patients. Most of our study group showed normal values indicating normal thyroid function except gastrointestinal tract cancer patients. Preserving normal activity of the thyroid gland is essential to many functions in the human body, especially those involved in metabolism and immune response. This study had some limitations, for example, the small size of the study group, and no data to reveal if patients had received any prescriptions that promote or suppress pituitary or thyroid gland in the previous 12 months. Increasing the number of study groups and acquiring the prescription history of the patients can help to strengthen the results.
Acknowledgements
The authors would like to thank the deanship of scientific research for their financial support for this project (project no. 1-440-6143). And thanks to all medical staff in king Faisal hospital and everyone participates in this study.
References
- Jabari M, Mirza A S, Tatwany A H, Alkhalaf K M, Alotaibi A H, Alrajhi M M, Al-Shehri H, Al-Faris A, Al-Sayed M. Effects of Growth Hormone (GH)Therapy on Free Thyroxine (FT4) And Thyroid Stimulating Hormone (TSH) Concentration in Children. Int. J. Pharm. Phytopharm. Res. 2017; 7(4): 13-17.
- Al-Maathidy A, Alzyoud J A M, Al-Dalaen S, Al-Qtaitat A. Histological alterations in the Thyroid Follicular cells induced by lead acetate toxicity in adult male albino rats Int. J. Pharm. Phytopharm. Res. 2019; 9(5): 19-26.
- Al-Bishri W M. Toxicity study of gold and silver nanoparticles on experimental animals. Pharmacophores. 2018; 9(1): 48-55.
- Kutia I M, Kopytsya M P, Hilova Y V, Petyunina O V, Berezin A E. The vascular endothelial growth factor-A gene polymorphism predicts clinical outcomes among acute ST-segment elevation myocardial infarction patients. Pharmacophores. 2020; 11(1): 100-11
- Mullur, R., Liu, Y. Y., Brent, G. A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014; 94(2):355-382. doi:10.1152/physrev.00030.2013
- Nilsson, M. & Fagman, H. Development of the thyroid gland. Development (Cambridge) 2017; 144(12):2123-40. doi:10.1242/dev.145615
- Dayan, C. M. Interpretation of thyroid function tests. Lancet, 2001; 357(9256):619-624. doi:10.1016/S0140-6736(00)04060-5
- Sheehan, M. T. Biochemical testing of the thyroid: TSH is the best and, oftentimes, only test needed - A review for primary care. Clinical Medicine and Research, 2016; 14, 83–92.
- Lin HY, Chin YT, Yang YC, Lai HY, Whang‐Peng J, Liu LF, Tang HY, Davis PJ. Thyroid Hormone, Cancer, and Apoptosis. Comprehensive Physiology, 2016; 6, 1221–1237.
- Liu, Z., Wang, F., Chen, X. Integrin αvβ3-targeted cancer therapy. Drug Development Research, 2008; 69, 329–339.
- Hodkinson, C. F., Simpson, E. E., Beattie, J. H., O'Connor, J. M., Campbell, D. J., Strain, J. J., Wallace, J. M. Preliminary evidence of immune function modulation by thyroid hormones in healthy men and women aged 55-70 years. J. Endocrinol. 2009; 202, 55–63.
- De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid, 2011; 21, 879–890.
- Khan SR, Chaker L, Ruiter R, Aerts JG, Hofman A, Dehghan A, Franco OH, Stricker BH, Peeters RP. Thyroid function and cancer risk: The Rotterdam study. J. Clin. Endocrinol. Metab. 2016; 101, 5030–5036.
- Tosovic A, Becker C, Bondeson AG, Bondeson L, Ericsson UB, Malm J, Manjer J. Prospectively measured thyroid hormones and thyroid peroxidase antibodies in relation to breast cancer risk. Int. J. Cancer, 2012; 131, 2126–2133.
- Søgaard M, Farkas DK, Ehrenstein V, Jørgensen JO, Dekkers OM, Sørensen HT. Hypothyroidism and hyperthyroidism and breast cancer risk: A nationwide cohort study. Eur. J. Endocrinol. 2016; 174(4):409-414. doi:10.1530/EJE-15-0989
- Boursi, B., Haynes, K., Mamtani, R., Yang, Y. X. Thyroid dysfunction, thyroid hormone replacement and colorectal cancer risk. J. Natl. Cancer Inst. 2015. doi:10.1093/jnci/djv084
- Seebacher V, Hofstetter G, Polterauer S, Reinthaller A, Grimm C, Schwameis R, Taucher S, Wagener A, Marth C, Concin N. Does thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trial. Br. J. Cancer, 2013; 109, 215–218.
- MELLEMGAARD, A., FROM, G., JØRGENSEN, T., JOHANSEN, C., OLSEN, J.H., PERRILD, H. Cancer risk in individuals with benign thyroid disorders. Thyroid, 1998; 8, 751–754.
- Bailey EB, Tantravahi SK, Poole A, Agarwal AM, Straubhar AM, Batten JA, Patel SB, Wells CE, Stenehjem DD, Agarwal N. Correlation of degree of hypothyroidism with survival outcomes in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor receptor tyrosine kinase inhibitors. Clin. Genitourin. Cancer, 2015; 13, e131–e137.
- Rasool M, Naseer MI, Zaigham K, Malik A, Riaz N, Alam R, Manan A, Sheikh IA, Asif M. Comparative study of alterations in tri-iodothyronine (T3) and thyroxine (T4) hormone levels in breast and ovarian cancer. Pakistan J. Med. Sci. 2014; 30, 1356–1360.
- Chan YX, Knuiman MW, Divitini ML, Brown SJ, Walsh J, Yeap BB. Lower TSH and higher free thyroxine predict incidence of prostate but not breast, colorectal or lung cancer. Eur. J. Endocrinol. 2017; 177, 297–308.
- Krashin, E., Piekiełko-Witkowska, A., Ellis, M., Ashur-Fabian, O. Thyroid hormones and cancer: A comprehensive review of preclinical and clinical studies. Front. Endocrinol. (Lausanne). 2019; 10, 59.
- Klein, J. R. The immune system as a regulator of thyroid hormone activity. Experimental Biology and Medicine, 2006; 231, 229–236.
- Almehmadi, M. M., Salih, M. M., Al-Hazmi, A. S. Awareness of human papillomavirus infection complications, cervical cancer, and vaccine among the saudi population: A cross-sectional survey. Saudi Med. J. 2019; 40, 555–559.
- Varghese, S., Montufar-Solis, D., Vincent, B. H., Klein, J. R. Virus infection activates thyroid stimulating hormone synthesis in intestinal epithelial cells. J. Cell. Biochem. 2008; 105, 271–276.
- Iams WT, Hames ML, Tsai JP, Dahlman KB, Talbott M, Richards KL, Reddy NM. Thyroid Dysfunction in Patients with Diffuse Large B-Cell Lymphoma Receiving Lenalidomide May be Mediated By TNF-α. Blood, 2014; 124, 4438–4438.
- Ghalaut VS, Yadav S, Ghalaut PS, Yadav A, Sachdeva A, Yadav R, Sharma TK, Shankar V. Association of Insulin like Growth Factor-1 (IGF-1) and thyroid hormones in patients of acute leukemia. Clin. Lab. 2012; 58, 227–231.
- Dalamaga, M., Karmaniolas, K., Papadavid, E., Pelecanos, N., Migdalis, I. Association of thyroid disease and thyroid autoimmunity with multiple myeloma risk: A case-control study. Leuk. Lymphoma, 2008; 49, 1545–1552.
- Evers, A. S. Paracrine interactions of thyroid hormones and thyroid stimulation hormone in the female reproductive tract have an impact on female fertility. Frontiers in Endocrinology, 2012. 3: 50. doi:10.3389/fendo.2012.00050
- Turkyilmaz, A., Eroglu, A., Aydin, Y., Yilmaz, Ö., Karaoglanoglu, N. A new risk factor in oesophageal cancer aetiology: Hyperthyroidism. Acta Chir. Belg. 2010; 110, 533–536.
- Massolt ET, Salih M, Beukhof CM, Kam BL, Burger JW, Visser WE, Hoorn EJ, Peeters RP. Effects of Thyroid Hormone on Urinary Concentrating Ability. Eur. Thyroid J. 2017;6, 238–242.
- Nelson, M., Hercbergs, A., Rybicki, L., Strome, M. Association between development of hypothyroidism and improved survival in patients with head and neck cancer. Arch. Otolaryngol. - Head Neck Surg. 2006; 132, 1041–1046.
- Smith GL, Smith BD, Garden AS, Rosenthal DI, Sherman SI, Morrison WH, Schwartz DL, Weber RS, Buchholz TA. Hypothyroidism in older patients with head and neck cancer after treatment with radiation: A population-based study. Head Neck, 2009; 31, 1031–1038.
- Patil VM, Noronha V, Joshi A, Bhattacharjee A, Goel A, Talreja V, Chandrasekharan A, Pande N, Mandal T, Ramaswamy A, Prabhash K. Influence of Hypothyroidism After Chemoradiation on Outcomes in Head and Neck Cancer. Clinical Oncology, 2018; 30, 675.
- Pearce, E. N. Thyroid dysfunction in perimenopausal and postmenopausal women. Menopause International. 2007; 13(1): 8-13. doi:10.1258/175404507780456746
- Kuijpens JL, Nyklíčtek I, Louwman MW, Weetman TA, Pop VJ, Coebergh JW. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid, 2005; 15(11):1253-1259. doi:10.1089/thy.2005.15.1253.
Contact SPER Publications
SPER Publications and
Solutions Pvt. Ltd.
HD - 236,
Near The Shri Ram Millenium School,
Sector 135,
Noida-Greater Noida Expressway,
Noida-201301 [Delhi-NCR] India